DNA damage response: reaching out of the nucleus
DNA damage is one of the most deleterious forms of cellular damage. If unrepaired, DNA lesions can create genomic instability, a hallmark of cancer 1. As most of the DNA is confined to the nucleus, for many years the study of DNA damage response has mainly focused on its nuclear effects, including mechanisms such as DNA repair and cell cycle arrest. The nucleus could be then seen like a closed box, where the impact of DNA damage would be only restricted to this organelle (Figure 1). Recently, the view of the cell has changed, subjects such as systems biology helped to integrate pathways that seemed to be otherwise unrelated and this allowed a view of the cell where communication is always occurring.
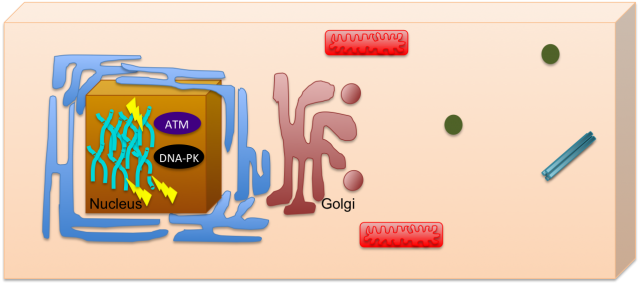
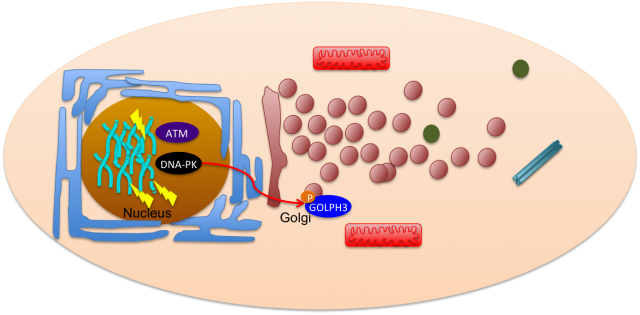
Pursuing this idea of constant interaction, many groups have recently studied how DNA damage can impact in other cell mechanisms not related with the classical nuclear effects. This is the case of Farber-Katz and co-workers who have found that DNA damage impacts on Golgi organization 2. They have shown that treatment of cells with camptothecin (CPT), doxorubicin (DOXO) or ionizing radiation (IR), all DNA damaging agents, led to Golgi dispersion which can be already observed 4h after treatment and increases over 24h. Although, the Golgi was found to be dispersed, this did not compromise its integrity, meaning that protein markers for cis- and trans-Golgi still colocalized. With this in mind, the authors went on and tried to investigate why Golgi dispersal occurred in response to DNA damage. Could it be due to apoptosis, since Golgi morphology changes when cells undergo this cell death process? The answer was no, non-apoptotic cells displayed dispersed Golgi after DNA damage. The authors also looked into the impact of microtubule depolymerization and cell cycle arrest, but none of these mechanisms had a role on Golgi dispersal. What about the proteins required to maintain Golgi morphology (GOLPH3, MYO18A and F-actin)? Yes, these factors are implicated in Golgi dispersal after DNA damage. Furthermore, DNA damage impairs trafficking from Golgi to the plasma membrane, a role for which the pathway GOLPH3/MYO18A/F-actin is highly important.
DNA damage response relies on cascade signaling that ultimately leads to repair. This signaling is mastered by three key protein kinases: ATM, ATR and DNA-PK. These kinases specifically phosphorylate SQ/TQ motifs on target proteins in order to activate them. Interestingly, GOLPH3, one of the Golgi proteins mentioned above, harbors a TQ and a TVQ site. To test whether these phosphorylation sites would be necessary for Golgi dispersal after DNA damage, Farber-Katz and colleagues transfected cells with constructs expressing either wild type GOLPH3, the unphosphorylated AQ-AVQ or the phosphomimetic EQ-EVQ. By treating the cells with inhibitors for the DNA repair kinases, the authors were able to conclude that DNA-PK is the one required for Golgi dipersal after DNA damage. In fact, DNA-PK was also found to directly interact with GOLPH3, further strengthening the idea that this kinase is responsible for the phosphorylation of GOLPH3 in response to DNA damage.
But why is this communication between nucleus and Golgi so important? To understand that Farber-Katz and co-workers depleted GOLPH3 or MYO18A from cells exposed to DOXO and have found that depleting these proteins led to an increase in the level of apoptosis as compared to untreated cells. Furthermore, overexpression of GOLPH3 rendered the cells more resistant to DNA damaging agents. Curiously, GOLPH3 was previously identified as a potent oncogene, which is overexpressed in cancer 3. Understanding, the effect of DNA damaging agents on GOLPH3 may also help to comprenend why many cancers where GOLPH3 has been found to be overexpressed have pour prognosis, i.e., cancer tends to progress regardless of chemotherapeutical treatment. Thus, knowing how a type of damage impacts on the whole cell is essential in order to better be able to prevent and conteract the development of tumorigenesis.
So maybe our initial boxed cell is more an interactive environment where there is a constant communication between distinct organelles in order to prevent cells from damage and allow to restore homeostasis (Figure 2).
References
- Hanahan and Weinberg 2011. Hallmarks of Cancer: The Next Generation. Cell 144 March. doi: 10.1016/j.cell.2011.02.013. ↩
- Farber-Katz S., Matthew D. Buschman, Marshall C. Peterman, Mengke Xing, Christopher J. Noakes, John Tat, Michelle M. Ng, Juliati Rahajeng, David M. Cowan & Greg J. Fuchs & (2014). DNA Damage Triggers Golgi Dispersal via DNA-PK and GOLPH3, Cell, 156 (3) 413-427. DOI: http://dx.doi.org/10.1016/j.cell.2013.12.023 ↩
- Scott et al 2009. GOLPH3 modulates mTOR signalling and rapamycin sensitivity in cancer. Nature 459 June. PMID: 19553991. doi:10.1038/nature08109 ↩
1 comment
[…] DNA damage response: reaching out of the nucleus […]