Baker’s Yeast Against Pain: Alkaloids production from glucose

Some pharmaceutical painkillers and analgesics like Noscapine, papaverine and tubocurarine or the most famous opioids codeine or morphine are Benzylisoquinoline derivatives. Few of them are used daily for thousands of people to relieve a variety of physical pains. However, due to their rather complex biosynthesis, they are still obtained by processing plant extracts (mainly from opium poppy) with relatively low global yields instead of being produced by organic synthesis, which not always this is possible or quite expensively. Benzylisoquinoline alkaloids (BIAs) have a common biosynthetic pathway that in plants derives from L-Tyrosine and has, among others, dopamine and S-reticuline as intermediates.
A common approach to the large-scale production of metabolites with commercial or therapeutic value, overcoming the different limitations of manufacturing using vegetable extracts or organic synthesis, has been its production in microbes. The use of increasingly advanced genetic engineering techniques allowed adapting and introducing specific plant biosynthetic pathways in microorganisms. However, in the case of the BIAs production, only the early intermediate S-reticuline, which is still far from the desired final molecules, has been achieved in Escherichia coli using glucose as a source.
Instead of using bacteria, which as prokaryotes lack many biosynthetic pathways and molecular machinery present and shared in eukaryotes, some results using genetic engineering in baker’s yeast have been encouraging. Until not long ago they were able to obtain, among other narcotics and antitussives, the precious morphine and codeine by feeding yeast with expensive molecules of late intermediate steps in the branches of BIAs biosynthetic pathway. Nevertheless, the implementation of the first steps in that pathway from L-Tyrosine to S-Reticuline remained elusive in yeast. The reason appears to be twofold. On one hand, yeast has a natural incapacity to hydroxylate L-tyrosine and it seem to be difficult making work exogenous tyrosine hydroxylases for metabolizing L-tyrosine to L-DOPA. On the other hand, we find the low catalytic efficiency of the norcoclaurine synthase (NCS) once it is introduced in yeast. This activity is necessary for generating the precursor of S-reticuline from dopamine (Figure 1).
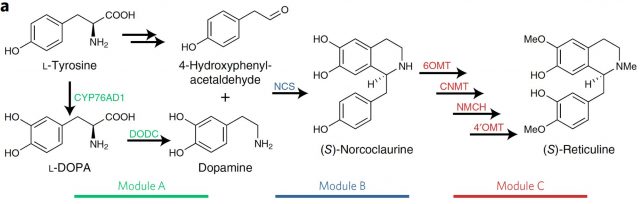
Therefore, here the two key steps to be solved for producing S-reticuline in yeast: first, to find a tyrosine hydroxylase activity compatible with yeast and then to improve the performance of the NCS. Since to find it is necessary to perceive, what William C. DeLoache and colleagues have done1 is to implement an easy-to-read biosensor which allowed them to find a yeast-active tyrosine hydroxylase1. This biosensor is a plant enzyme DOPA dioxygenase (DOD) that converts L-DOPA into betaxanthin pigment, which is nicely fluorescent (Figure 2).
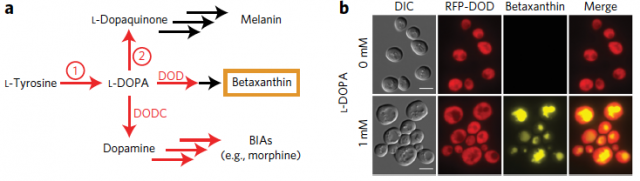
The fluorescence produced by this biosensor (here the DOD variant from four o’clock flower, Mirabilis jalapa) is proportional to the amount of L-DOPA present in the culture. Thus, it potentially allowed them not only to screen for tyrosine hydroxylase activities active in yeast but also, once found a suitable enzyme, to explore different mutations that improve on its purpose. In this way, they found that the tyrosine hydroxylase from the sugar beet B. vulgaris (named CYP76AD1) produced enough L-DOPA to be transformed for the biosensor in a quantity of betaxanthin detectable by naked eyes.
Afterwards, rounds of mutagenesis and DNA shuffling on CYP76AD1 native sequence with the subsequent screenings detected a CYP76AD1 double mutant three to four-fold more efficient producing L-DOPA than the wild-type enzyme from sugar beet plants. Its activity increased to the extent that it was needed to introduce a mutant Aro4p enzyme that increases the intracellular L-tyrosine concentration because it activity is not inhibited by a product-dependent loop. CYP76AD1 double mutant increased activity seems to be, at least in part, due to a highest expression level and to a considerable reduction in its undesirable DOPA oxidase activity. Making possible that less L-DOPA is diverted in unprofitable melanin production. After all, the additional expression of DOPA decarboxylase (DODC) from the Gram-negative bacterium Pseudomonas putida yielded considerable production of dopamine just growing that strain two days in medium with 4% glucose (Figure 2).
Having the first step solved with a yeast strain that was able to produce dopamine, the following problematic steps in the synthesis of S-reticuline were addressed searching through different norcoclaurine synthase (NCS) plant genes. Finally both, the production of S-norcoclaurine from dopamine (by the NCS), as the S-reticuline from S-norcoclaurine (by four other enzymes) was achieved by introducing in that yeast strain the necessary enzymes from P. somniferum (opium poppy). (Figure 1).
However, though promising and key achievement, S-reticuline production from glucose using yeast was still far from the main goal, which is to generate opioid compounds with a palliative and therapeutic value from simple carbon sources like sugar. The pathway from S-reticuline to those valuable opioids includes many complicated steps and branches that baker´s yeast do not fulfil alone with its enzymatic repertoire. Overcoming this, just bit more than three months after DeLoache published the work explained above, Smolke´s group in Stanford brought in 2 a striking work. Waving a handful of common tools in genetic engineering and enzyme improvement they managed to make work together in a yeast strain diverse enzymatic activities (up to 23 different ones) coming from plants, mammals, bacteria and the proper yeast; achieving the complete biosynthesis of thebaine and hydrocodone (two potent codeine related compounds) starting from sugar.
Therefore, as actions speak louder, it seems feasible to produce expensive opioids through metabolizing cheap sugar using baker’s yeast. So far this two works are proof of principle that we have the tools. But the major drawbacks in both jobs I have not mentioned so far are the low concentrations of final compounds obtained. These amounts are still very far from being commercially competitive. Meaning that it is necessary to work hard on polishing the artificial biosynthetic pathways built in yeast to increase its performance and large-scale yield.
Regardless of the time it takes us to improve it the promised benefits are many. Among them, the faster, cheaper and easier production (compared with the actual setting that depends on the annual poppy crop) will promote lower prices of substances that contribute to cure or palliate the suffering of millions of people. But quite likely, it will also bring disadvantages. For the moment, the strains generated on these works and others are almost useless for effective production even using huge large-scale bioreactors. But at the moment those strains are available, the assembly required for home brewing is away less difficult to maintain than any opium poppy culture setting, which could lead to an illicit use of yeast-obtained opioids. Therefore, further legal proceedings will probably be necessary to ensure people receiving the benefits without getting the disadvantages.
References
- DeLoache, W. C. et al. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat. Chem. Biol. (2015). doi:10.1038/nchembio.1816 ↩
- Galanie, S., Thodey, K., Trenchard, I. J., Interrante, F. & Smolke, C. D. (2015) Complete biosynthesis of opioids in yeast. Science DOI: 10.1126/science.aac9373 ↩
1 comment
[…] Las levaduras pueden tener muchos usos. Uno fascinante, y que a la policía y a algún “comerciante” pondrá los ojos como platos, puede ser la producción de sustancias contra el dolor (alcaloides) a partir de un azúcar. Daniel Moreno […]