Graphene Oxide: Yet another “cancer cure of the week”?
Graphene Oxide: Yet another “cancer cure of the week”?
Since its discovery in 2003, graphene has become one of the “materials of the future”, with applications ranging from tissue engineering to energy storage. Recently, a paper published in Oncotarget 1 by researchers at the University of Manchester has shown its potential in anti-cancer therapy, in addition to its already known applications in medicine as a drug delivery agent.2
Graphene, formed by two-dimensional sheets of carbon atoms without any additional functional groups, does not form stable dispersions in water or other biologically relevant solvents.3 Instead, graphene oxide is the alternative generally used for biological experiments.4 It is a water-soluble derivative of graphene that can be more easily manipulated in biological systems and has already shown to be non-toxic for normal stem cells; in fact it promotes their differentiation towards multiple cell lineages including neurons, chondrocytes and adipocytes.56These properties, that are already being exploited for tissue engineering and regenerative medicine, made the authors of this research wonder if graphene oxide could be used in order to selectively inhibit the proliferative expansion of cancer stem cells by inducing their differentiation towards “bulk” (non-stem) cancer cells.
Cancer stem cells are resistant to conventional therapeutic approaches 78such as chemo and radiotherapy. For this reason, they have been directly implicated in tumour recurrence and distant metastasis.910However, there is still some controversy about their origin and function, mostly due to not having been fully investigated yet. For example, it is not well known whether they originate from normal stem cells, or from mature cancer cells that undergo “de-differentiation”. What we know so far is, cancer stem cells are not necessarily tumour initiators, but they are defined by their existence within growing tumours as well as by their ability to propagate and regenerate them. 11
For this study, the researchers tested the effect of the presence of graphene oxide flakes on the formation of “oncospheres”. These experiments are based on the fact that, while non-stem cancer cells undergo anoikis (a form of apoptosis after being detached from their surrounding extra-cellular matrix) when cultured in suspension, a characteristic of cancer stem cells is their ability to initiate tumours and to undergo anchorage-independent growth under these conditions.12 Thus, they proliferate and form three-dimensional spherical structures, each of them originating from the clonal proliferation of a single stem cell and not due to the aggregation of non-stem cancer cells.
Initially, the activity of graphene oxide was tested against the proliferation of breast cancer stem cells. Two size ranges -either smaller or larger than the target cells- of graphene oxide flakes were tested, and both sizes showed a dose-dependent inhibition of oncosphere formation, with similar potency (Figure 1). Since the larger flakes were bigger than a single cell and therefore could not be internalised, it was concluded that the graphene oxide must be working at the cell surface. The observation that the CD24 protein was being expressed at a high rate on cancer stem cells after being treated with graphene oxide, was consistent with this hypothesis, as CD24 is a cell surface protein and, therefore, it is likely to be affected by an agent exerting its effects on the cell surface.
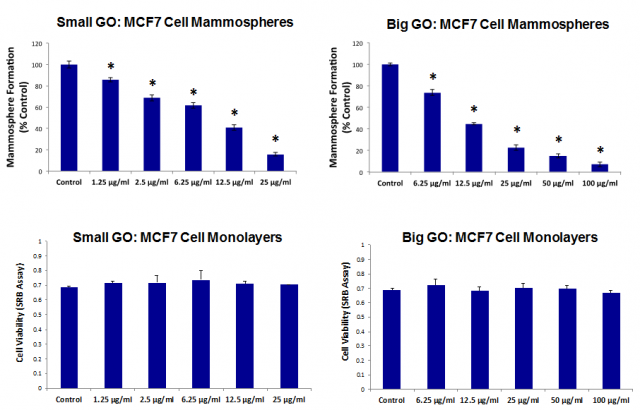
The CD24 protein is present in a variety of cancer cells, and its expression is involved in tumour progression and metastasis. Since it is mainly expressed on immature cells and usually absent from cells that have reached their differentiation stage, the experiments suggested that graphene oxide inhibits oncosphere formation (i.e. cancer stem cell proliferation) by promoting the differentiation towards mature cancer cells. To put it otherwise, graphene oxide does not reduce the number of cancer stem cells by “killing” them but, on the contrary, by inducing their maturation towards bulk cancer cells.

With these results in hand, graphene oxide was subsequently evaluated against other cancer stem cell lines from multiple cancer types, and it showed to also effectively inhibit oncosphere formation across ovarian, prostate, lung and pancreatic cancers, as well as glioblastoma (brain). This indicates that it must be targeting a relatively specific and highly-conserved phenotypic property of cancer stem cells across multiple cancer types. Observing that the viability of non stem cells from these five cancer cell lines was not affected, highlighted the specificity of graphene oxide for cancer stem cells. Further experiments on normal skin cells showed that graphene oxide did not affect their viability either. This is consistent with the previous findings of other research groups about its lack of toxicity for normal cells.13
The inability to treat metastasis keeps being the major source of cancer related deaths, and its prospective treatment with graphene oxide is far from being a miracle cure for the reasons exposed above, since there is still much research left to do towards the full understanding of cancer stem cells. Besides, there are tumour types in which the existence of cancer stem cells as a “proper” group of cells with common features is not clear yet, thus making difficult the use of specific drugs to target them. However, these findings have shown graphene oxide as another promising anti-cancer agent we should take into account for some types of tumours where cancer stem cells have showed to be relevant and therefore a good target for metastasis prevention.
References
- Fiorillo, M., et al. Graphene oxide selectively targets cancer stem cells, across multiple tumour types: Implications for non-toxic cancer treatment, via “differentiation-based nano-therapy”. Oncotarget, 2015;6(6), 3553. ↩
- Liu, Z., et al. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. Journal of the American Chemical Society. 2008; 130(33), 10876. ↩
- Hernandez, Y., et al. Measurement of Multicomponent Solubility Parameters for Graphene Facilitates Solvent Discovery. Langmuir. 2010;26(5), 3208. ↩
- Dreyer, D. R., et al. The chemistry of graphene oxide. Chem Soc Rev. 2010; 39(1), 228. ↩
- Bressan, E., et al. Graphene based scaffolds effects on stem cells commitment. Journal of translational medicine. 2014; 12(1), 296. ↩
- Lee, W.C., et al. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS nano. 2011; 5(9),7334. ↩
- Sinha, N, et al. Relevance of cancer initiating/stem cells in carcinogenesis and therapy resistance in oral cancer. Oral oncology. 2013; 49(9), 854. ↩
- Easwaran, H. et al. Cancer epigenetics: tumour heterogeneity, plasticity of stem-like states, and drug resistance. Molecular cell. 2014; 54(5), 716. ↩
- Dawood, S., et al. Cancer Stem Cells: Implications for Cancer Therapy. Oncology. 2014; 28(12). ↩
- Colak, S., et al. Cancer stem cells—important players in tumour therapy resistance. The FEBS journal. 2014; 281(21):4779. ↩
- White, A. C., et al. Refining the role for adult stem cells as cancer cells of origin. Trends Cell Biol. 2015;25(1):11. ↩
- Magee J.A., et al. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer cell. 2012; 21(3), 283. ↩
- Liao K.H., et al. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS applied materials & interfaces, 2011; 3(7), 2607. ↩