From venoms to drugs

Animals like spiders, snakes, scorpions, and cone snails inject venoms to their preys to immobilize or kill them. But what there is in these venoms that can trigger such a strong effect? The venom of these animals is actually a cocktail of toxins, from which one of the main components are peptides. Due to their strong effects, there is no wonder that the active components of the venoms have attracted a lot of attention from scientist to be use as drug leads. Many research groups in the world center their explore different possibilities in order to go “from venoms to drugs”. One therapeutic use of the toxins of the venoms is as analgesics, or painkillers.
The snake black mamba (Dendroapsis polylepis) injects a venom in their preys that acts very quickly disrupting the central nervous system and causing respiratory paralysis, as well as a wide range of other physiologically important receptors. If not treated with antivenom a human could die within 30 minutes of envenomation. The potency of the black mamba venom stimulated researchers from the CNRS in France 1 to subject mamba venom through a screen designed to discover new blockers of the acid-sensing ion channels (ASICs), which are main players in the pain pathway. ASICs are associated with a range of physiological conditions including inflammation, ischemia and physical trauma. In their screening, Diochot et al performed electrophysiology on rat ASIC1a expressed in Xenopus oocytes and observed that black mamba venom reversible inhibits rat ASIC1a current. The peptides mambalgin-1 and mambalgins-2 were isolated as the active components of the mamba venom.
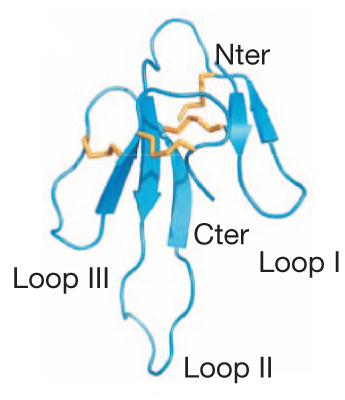
Mambalgin-1 and mambalgin-2 only differ by one residue at position 4. They are composed by 57 amino acids with eight cysteine residues that form four disulfide bonds, dividing the peptide in three loops (Figure 2). Mambalgins are members of the three-finger toxin family. A three-dimensional structure model shows a triple-stranded and short double-stranded antiparallel -sheets connecting loops II and III, and loop I, respectively, the three loops emerging from the core of the toxin like fingers from a palm. The molecule presents a strong positive electrostatic potential that seems to be important for its biological activity. Due to their high similitude only mambalgin-1 was used in the experiments to further characterize the biological activity of these molecules.
To elucidate the biological activity of the peptide, the authors used recombinant ASICs and rat pain models. They found out that mambalgins are potent, rapid, and reversible inhibitors with nanomolar potency of ASICs expressed either in central or peripheral neurons.
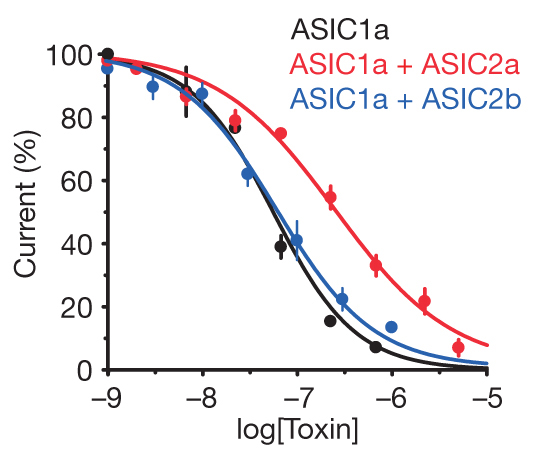
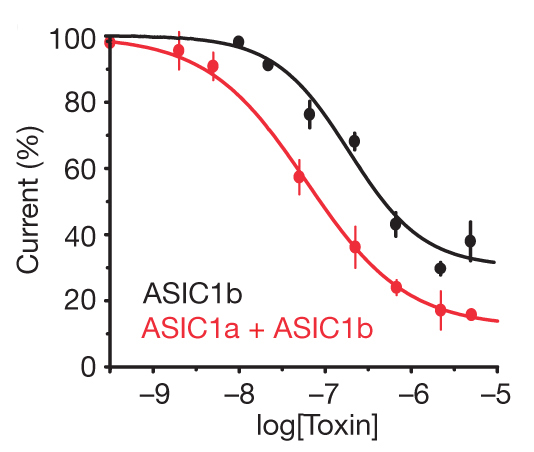
Electrophysiology experiments in COS-7 cells demonstrated that mambalgins inhibit homomeric ASIC1a and heteromeric ASIC1a + ASIC2a, and ASIC1a + ASIC2b, that is, all the ASIC channels expressed in the central nervous system (Figure 3). Furthermore, mambalgins inhibit ASIC1b and ASIC1a + ASIC1b channels, which are specific of sensory neurons (nociceptors) (Figure 4). Interestingly, mambalgins are very selective for ASICs and did not affect a range of other ion channels or receptors reported to be involved in analgesia. The selectivity of these molecules is therefore a remarkable strength.
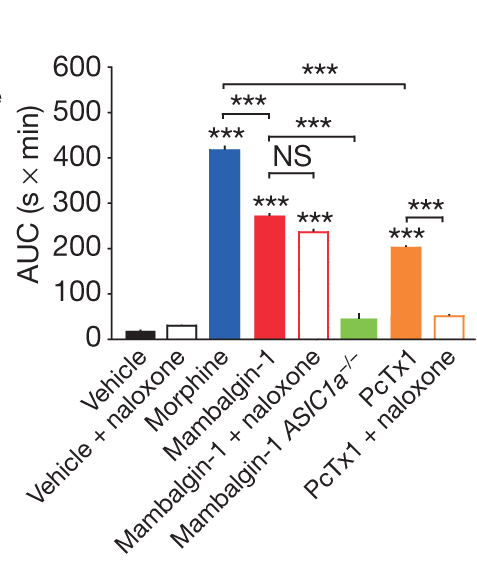
Importantly, mambalgins did not cause neurotoxic effects in mice. Diochot et al studied the analgesic effect of mambalgins in mice by using different methodologies. To determine the effect of mambaglins in acute pain, the authors measured the effects on acute thermal pain (46 °C) using the tail-immersion and paw-immersion tests. After intrathecal injection of the peptides the authors observed a large increase in response latencies. Knockout mice models showed that the analgesic effect was completely lost in ASIC1a knockout mice, demonstrating the essential implication of ASIC1a-containing channels. The analgesic effect of mambalgins was comparable with morphine. However, mambalgins were resistant to naloxone, a morphine antagonist (Figure 5), suggesting that mambalgins and morphine present a different mechanism of action. On contrary, the analgesic effect of PcTx1, a peptide from the tarantula spider venom, was inhibited by naloxone (Figure5).
The authors also measured the effect of mambalgins against inflammatory pain by inducing inflammatory hyperalgesia with intraplantar carrageenan and observed similar results.
Next, the authors compared the effects of mambalgins with morphine with more detail and found out that the central analgesic effect of mambalgins shows much less tolerance compared with morphine (Figure 6) and no respiratory distress (Figure ). Thus, mambalgins might result in an improved therapy for the treatment of pain compared with morphine.
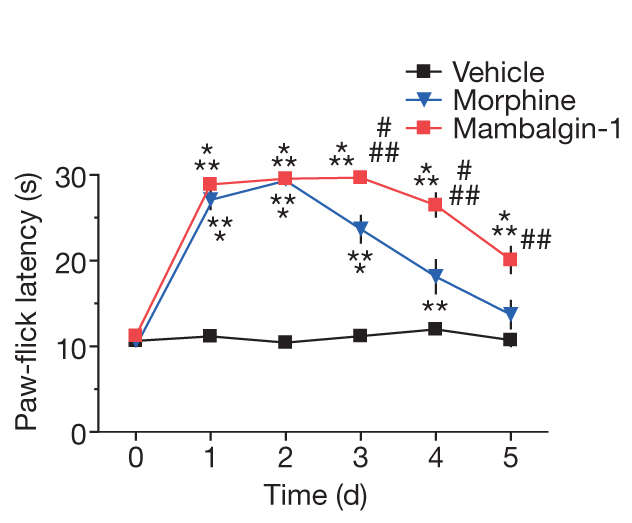
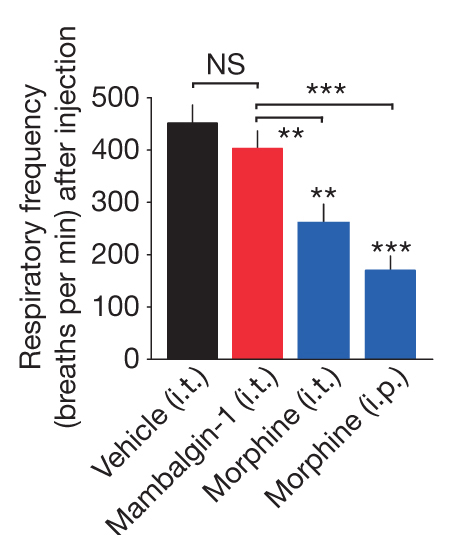
The work presented in this paper is very significant for the development of new treatments for pain, a condition that affects millions of people worldwide. So far mambalgins seem to be promising molecules to relief pain. Maybe nature will bring us with these natural peptides a new more efficient treatment for pain.
The use of peptides as drug leads is very extended in the pharmaceutical industry. However, peptides are not yet ideal drugs because they are not stable molecules and present very low oral bioavility, therefore they have to be delivered directly to the spinal cord. However, researchers are making a lot effort to increase the drug-like properties of peptides and in near future natural bioactive peptides like mambalgins could be in the market in a pill format treating conditions as widespread as pain and therefore improving the quality of life of the population.
Next time a snake cross in your way you will be aware that its venom contains precious molecules that exert beneficial biological activity, but don’t forget that its venom could kill you in 30 minutes!!
References
- Sylvie Diochot, Anne Baron, Miguel Salinas, Dominique Douguet, Sabine Scarzello, Anne-Sophie Dabert-Gay, Delphine Debayle, Valérie Friend, Abdelkrim Alloui, Michel Lazdunski & Eric Lingueglia (2012) Black mamba venom peptides target acid-sensing ion channels to abolish pain Nature DOI: 10.1038/nature11494 ↩