Killing cancer without toxic drugs
“The dose makes the poison!” This adage, attributed to Paracelsus, explains a basic principle of toxicology: a substance can produce a harmful effect only if it reaches a certain “toxic” concentration.[1] In reverse, it also means that a substance is not harmful below this toxic dose, and can even be vitally important for a biological system. Conventional chemotherapeutics are an excellent example of this principle at work. Many cancer drugs on the market today lead to severe side effects because they are administered to the patient at the high doses required to destroy cancer cells, but, unfortunately, these doses are also toxic to healthy cells.
Over the past few decades, cancer has become a worldwide health issue with an ever-increasing growth rate of cancer patients each year.[2] Nowadays, the lifetime risk of developing or dying from cancer is steadily increasing as a result of longer life expectancy and changed living conditions. Therefore, cancer concerns humanity as a whole and constitutes one of the major public-health issue that research has to jump over in the near future.[2]
So far, the treatment of advanced localised tumours is provided by systematically administrated chemotherapy, radiotherapy or surgery or most of the time a combination of those three.[2] The classical cytostatic chemotherapeutic drugs do not show specific accumulation into tumour tissues but rather spread throughout the whole body and lead to systemic toxicity. This results in typical side effects as nausea, vomiting diarrhoea, nerve and organ damages, and bone marrow suppression that significantly limit the therapeutic outcome. Therefore, the ability to efficiently deliver a drug to a tumour site is a key issue of chemotherapy that can be addressed by using nanotechnology.[3-7]
Nanotechnology enables the creation of delivery vehicles able to overcome physiologically imposed barriers, allowing new approaches for reducing the unwanted side effects of systemic delivery, increasing targeting efficiency and so improving chemotherapy efficacy (Figure 1). These “nanocarriers“ are designed to navigate to a certain region of the body (through surface functionalization, size and shape manipulation, among others), loaded with toxic drugs that (theoretically) release at the target site. This Trojan Nano-Horse is required to disguise dangerous agents (e.g. drugs) to protect them from disruption by the immune system as they travel to tumour cells. Despite its many advantages, this system is imperfect: poor targeting, premature leakage, or build-up of nanocarrier residue leads also to serious side effects.
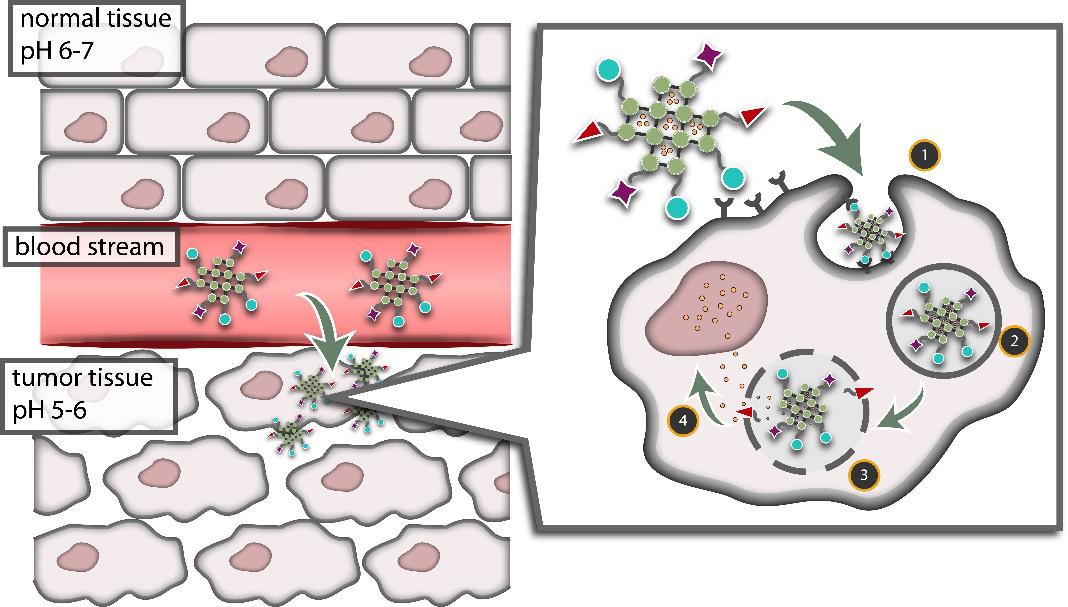
The goal of our research was to create a new class of chemotherapeutic materials that take advantage of the ability of healthy cells to regulate their intracellular dose of certain compounds more effectively than cancer cells. Iron-based nanoparticles were established to deliver iron ions to cells throughout the body (without adding any of the other toxic substances that nanocarriers are usually laced with and therefore circumventing the negative side effects associated with current chemotherapeutics).
Iron is one of the most important minerals in the human body. It is not only an integral component of the metabolic processes, including oxygen transport, but also for electron transport, and can be found in all cells throughout the human body. However, the iron ion is toxic to healthy cells above a threshold concentration as it can form free radicals. The only reason iron is not lethal to humans is because our cells have developed complex regulatory pathways (called feedback loops) that dictate how many iron ions are allowed to enter the cell and how quickly they must be removed. Consequently, the concentration of iron ions in human cells is controlled with a truly impressive degree of precision.
Unlike healthy cells, cancer cells consume ions and molecules at much higher rates that facilitate the rapid cell growth characteristic of cancer, but which do not allow for proper regulation. In other words, the cancer cell is programmed to bring ions and molecules into the cell instead of pumping them out, making the cancer cell incapable of maintaining a healthy internal balance.
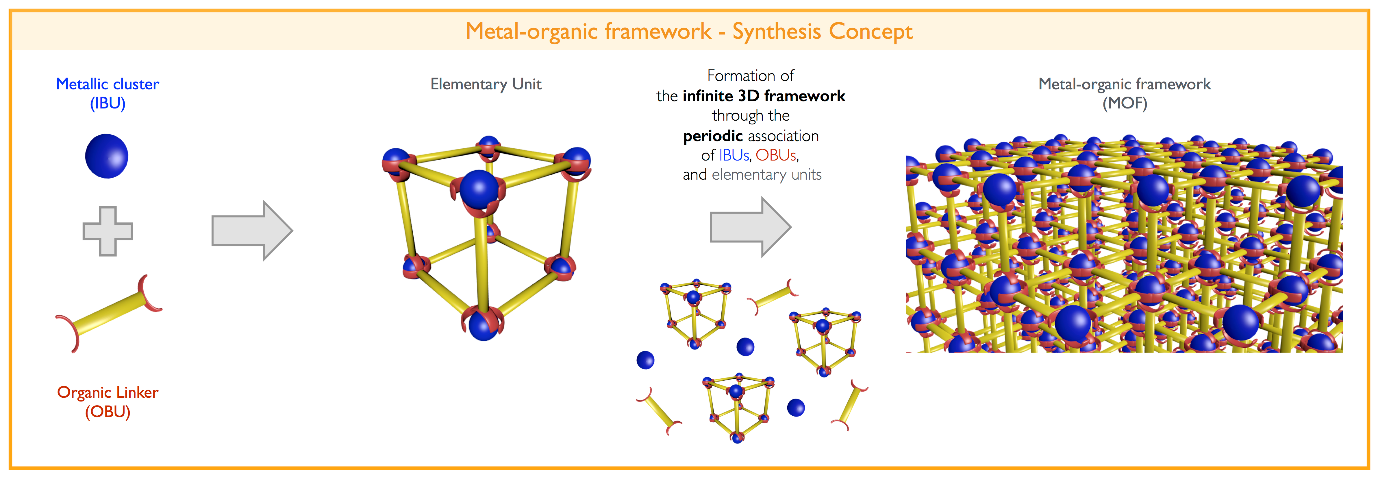
We synthesised iron metal-organic framework (MOF) nanoparticles that are designed to respond to the chemical differences between the intra- and extra-cellular environments and release iron ions when they have passed into the cells. MOFs are a uniquely valuable class of hybrid materials in that their structure and composition can be controlled at the atomic level. [8, 9] As the name suggests, MOFs are made of metal ion/cluster (inorganic) and carbon based (organic) molecular building units that assemble into crystalline solids (Figure 2). A variety of different organic and inorganic units have been identified to date, giving material scientists an incredible degree of freedom when designing new MOFs because we can mix and match familiar units to create new pairings, and allowing us to tweak our designs by switching out one unit for another until we make a material with exactly the features we desire. The control we can exercise over MOFs makes them ideal candidates for our novel nanoparticle delivery system.[6,7,10]
Our smart iron-based MOF nanoparticles address all shortcomings of current nanocarries by using exclusively non-toxic components, giving our particles the power to eradicate cancerous cells without affecting healthy ones – Killing cancer without being toxic!
Unlike traditional nanocarriers which are just vessels loaded with drug cargo, we have synthesized nanoparticles that are both the cargo and the carrier – a nanocarrier without any toxic substances. The nanocarrier is uptaken by cancer cell and slowly degraded into its building blocks (Figure 3). The degradation products causes pyroptosis, a programmed cell death, in which the cell membrane is perforated (a cell “explosion“ can be overserved under the microscope, Figure 4). [12]
Video. Iron-based MOF nanoparticles with dye molecules were incubated with cancer cells. Directly after incubation, the particles are not visible as the dyes are trapped within the nanoparticles. About 40 h after incubation, dye was visible indicating that the iron-based MOF nanoparticles have degraded. Shortly afterwards, we observed a sudden spread of the dye all over the cell followed by a burst and “explosion“ of the entire cell.[12]
This novel concept of nanoparticle-based therapy entirely without toxic substances may open up new ways of thinking about nanoparticles and their use in therapy. This solution (to kill cancer without cytotoxic drugs) will not only enable the expansion of nanotechnology into many other medical treatments, but will also help to decrease the cost of chemotherapy and reduce discomfort during cancer therapy. In addition, our approach may lead to the discovery of novel nanoparticle-based therapeutics and help to overcome the bottleneck that is currently strongly limiting the success of nanoparticles in therapy
Acknowledgements
We acknowledges funding from the Basque Government Industry and Education Departments under ELKARTEK and PIBA (PIBA-2018-06) programs, respectively and from the Spanish State Research Agency (AEI) and the European Regional Development Fund (ERFD) through the project PID2019-106099RB-43/AEI/10.13039/501100011033.
References
[1] Paracelsus, Das Buch Paragranum, CreateSpace Independent Publishing Platform 2013, ISBN-10: 1484049667.
[2] World Cancer Report 2014 edited by B. W. Steward and P. Wild, International Agency for Research on Cancer – World Health Organization, ISBN 978-92-832-0429-9.
[3] O. C. Farokhzad, R. Langer, ACS Nano 2009, 3, 16. https://doi.org/10.1021/nn900002m
[4] H. Goesmann, C. Feldmann, Angew. Chem. Int. Ed. 2010, 49, 1362. https://doi.org/10.1002/anie.200903053
[5] T. M. Allen, P. R. Cullis, Science 2004, 303, 1818. https://doi.org/10.1126/science.1095833
[6] R. Freund, U. Lächelt, T. Gruber, B. Rühle, S. Wuttke, ACS Nano 2018, 12, 2094-2105. https://doi.org/10.1021/acsnano.8b00932
[7] S. Wuttke, M. Lismont, A. Escudero, B. Rungtaweevoranit, W. J. Parak, Biomaterials 2017, 123, 172-183. https://doi.org/0.1016/j.biomaterials.2017.01.025
[8] H. Furukawa, K. E. Cordova, M. O’Keeffe, O. M. Yaghi, Science 2013, 341, 1230444. https://doi.org/10.1126/science.1230444
[9] J. Zhe, H. Wang, S. Canossa, S. Wuttke, O. M. Yaghi, Adv. Funct. Mater. 2020, 2000238. https://doi.org/10.1002/adfm.202000238
[10] E. Ploetz, H. Engelke, U. Lächelt, S. Wuttke, Adv. Funct. Mater. 2020, 1909062. https://doi.org/10.1002/adfm.201909062
[11] M. Limont, L. Dreesen, S. Wuttke, Adv. Funct. Mater. 2017, 27, 1606314. https://doi.org/10.1002/adfm.201606314
[12] E. Ploetz, A. Zimpel, V. Cauda, D. Bauer, D. C. Lamb, C. Haisch, S. Zahler, A. M. Vollmar, S. Wuttke, H. Engelke, Adv. Mater. 2020, 1907267. https://doi.org/10.1002/adma.201907267
Author:
Stefan Wuttke1,2
1BCMaterials, Basque Center for Materials, Applications and Nanostructures, PV/EHU Science Park, 48940 Leioa, Spain
2IKERBASQUE, Basque Foundation for Science, 48013 Bilbao, Spain