World’s smallest machines: Nobel Prize in Chemistry 2016
This year the Nobel Prize in Chemistry has been awarded to Jean-Pierre Sauvage, Fraser Stoddart and Ben Feringa “for the design and synthesis of molecular machines”1. These devices, often described as the tiniest “Lego”2, are indeed so small that is difficult to grasp their size used as we are to the macroscopic world. Molecular machines are in the nano-range, a thousand times smaller than a hair. They are made by one or few molecules linked together, comprising several hundred atoms. If a molecule can use the energy input (stimuli) that it receives to perform a mechanical movement (output) is termed molecular machine. But this is extremely challenging to achieve because molecules tend to move, spin, and rotate randomly, taking energy from the environment. So they have to be designed -and then synthesized- to move in a controlled way. That is precisely what this year´s winners of the Nobel Prize in Chemistry pioneered and excel at.
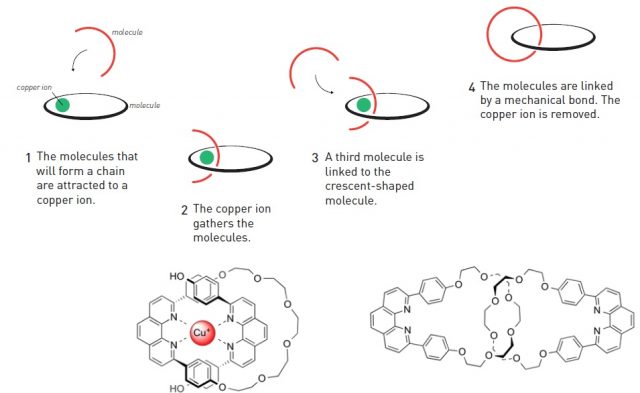
As an analogy with macroscopic world, the very basis of many molecular machines is that one part of the molecule moves from one position to another, while the other remains static (think of a bicycle pump for example). Obviously, that requires interlocking, at least, two molecules. Chemists usually link together atoms and molecules through covalent bonds, after which the molecules remain bonded one to another. This approach is obviously not suitable for back and forth movement. Nevertheless, molecules can also be held together through mechanical bonds. Some of the structures featuring this type of bond are catenanes (two interlocked rings) and rotaxanes (a ring locked into an axle by stoppers at the ends). Jean-Pierre Sauvage´s great contribution was to enable the first practical synthesis of catenanes. In order to interlock two rings, one has first to be open, inserted into the other and then closed. The key to his design was to use a copper ion as a template for bringing the two molecules together, thanks to the coordination of nitrogen atoms (present in these molecules) to the copper ion. When the two fragments were in place, the ring was closed by covalent bond formation and afterwards the copper ion was removed (Figure 1). Then one ring could move around the other.
Jean-Pierre Sauvage is considered to have laid the foundations for the development of the field, whereas Fraser Stoddart is regarded as the actual starter of the artificial molecular machinery with his discovery of the “molecular shuttle” in 1991 (Figure 2, up). In the rotaxane he designed the ring shifts between the two hydroquinone units, by random thermal motion. Even more important was the realization, in 1994, that by having two different binding sites the position of the ring could be controlled: The cationic ring (in dark blue), poor in electrons, prefers to sit on the benzidine group (in light blue), rich in electrons, rather than on the biphenol group, less rich (Figure 2, middle). However, protonation (or electrochemical oxidation) of the benzidine group (now in purple) makes it electron-poor, and thus the cationic ring moves to the biphenol group (Figure 2, down). This tunable system was the first example of controlled motion of a molecule over a molecular track.
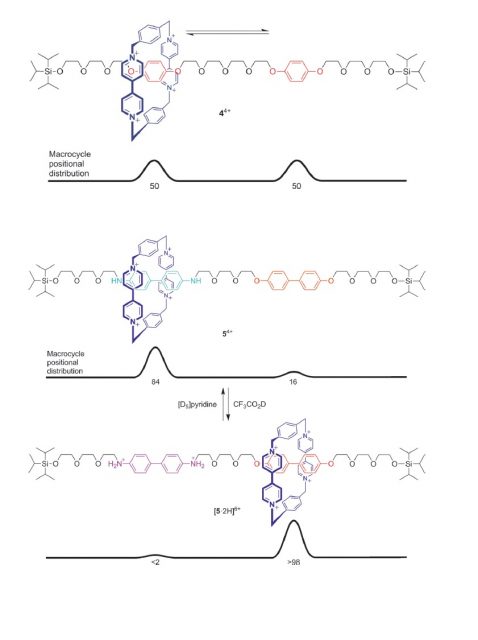
Since then, higher complexity molecules based on rotaxanes have been built by Stoddart and colleagues, including a “molecular elevator” which can move between two planes separated by 0.7 nanometres, as well as an “artificial muscle” which was able to bend a thin gold cantilever structure. Moreover, together with other researchers, they also developed a rotaxane-based computer chip with a 160 kB memory.
The last awarded of this year´s Noble Prize in Chemistry is Ben Feringa, for developing artificial molecular motors. These entities differ from previously shown systems in the way that there are not interlocked parts, but a single molecule. In 1999, the Dutch chemist designed a molecule with two paddles connected by a carbon-carbon double bond. Importantly, the unidirectionality in movement required to be called a motor was achieved because the design of the paddles did not allow free rotation around the axis. Only upon irradiation of UV light and subsequent heating they turn, endlessly as long as energy input is provided (Figure 3).
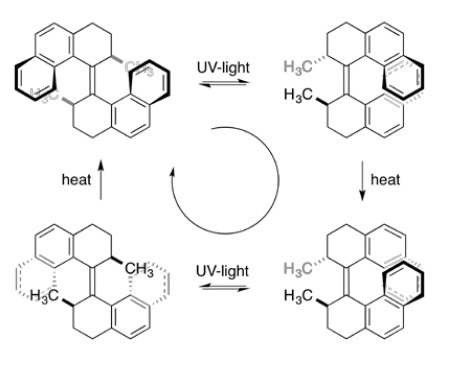
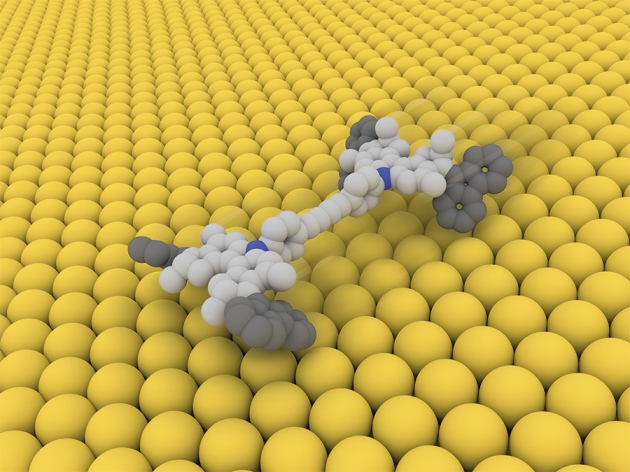
Modifications of the original motor have led, over the years to several improvements such as fast rotation, up to12 million revolutions per second! Motors have also been used to perform different tasks, such as switching the chirality of a catalyst and rotating a macroscopic object (a micron size stirrer) in a liquid-crystal medium. Even more impressive, visually powerful images, is to see a “nanocar”, with motors as wheels, moving over a copper surface (Figure 4, movie available here).
If the Noble Prize could be shared by four instead of only three persons David Leigh would probably have been awarded it. He is one of the leading scientists in finding applications for molecular machines, such as a small-molecule robotic arm that can pick-up, transport a release a cargo (a molecule in this case). Even more impressive is the synthesis of a molecular assembly line, in which the ring walks down the rotaxane picking up amino acids and synthesizing a short peptide chain. That is basically how ribosomes build proteins! Of course, this is still a very primitive analogue of it, but it shows the great potential of molecular machines. It has to be considered that molecular machines is a new, emerging field of chemistry. Thus, these devices, although impressive as they are, are still in their infancy. They cannot compete with nature’s “molecular machines”, exemplified by proteins such as ribosomes or myosin. Having said that, in the macroscopic world, at the time of building the first electrical machines they were also too simple devices with not foreseeable applications. However, in a lapse of fewer than 300 years they have completely revolutionized the world. This could also be the case with the nano-machines.
References
- O. Ramström, The Nobel Prize in Chemistry 2016. Scientific Background on the Nobel Prize in Chemistry 2016. Molecular Machines. ; b) O. Ramström, G. von Heijne, S.S Linse. The Nobel Prize in Chemistry 2016. Popular Science Background. ↩
- Peplow, M. Nature, 2015, 525, 18-23. DOI:10.1038/525018a ↩