Marginal macrophages and LXR: living on the edge
Our immune system comprises a great variety of cell types and each of these groups is specialized in separate and defined actions. Macrophages play an essential role within this defence system by enhancing the immune response. In particular, they are in charge of “swallowing” (technically speaking, fagocyting) waste products, foreign materials and microorganisms. In addition, macrophages secrete chemical compounds that predetermine the response of the rest of the components of the immune system.
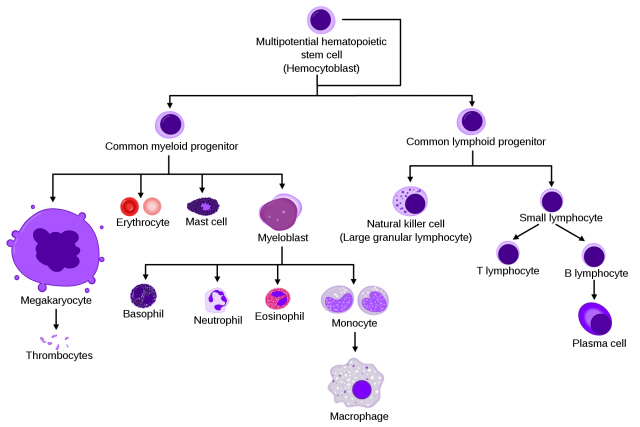
Macrophages are ubiquitously distributed throughout the body but are quite heterogeneous regarding their different locations. These variances allow identifying different macrophage types (“races”, if we wish to make an analogy with humans). This diversity also reflects functional heterogeneity, which seems to be determined, partly, by the environment of the organ where they exert their roles.
In this paper 1 Dr. Castrillo’s group focus their research in the study of macrophages of the spleen. More precisely, their work focuses on the use of multiple techniques in order to unravel the role of nuclear receptor LXR on certain spleen macrophage populations. This article illustrates the study of the function of a protein throughout its loss.
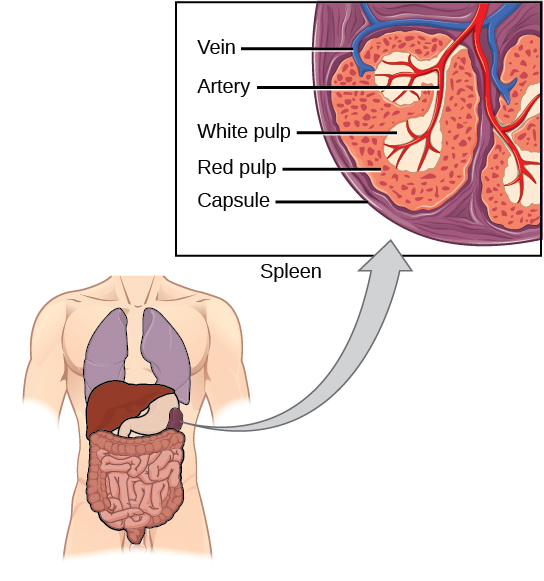
The spleen is in charge of blood filtering and maintenance and it also detects foreign bodies and substances. Macrophages play a key role on all those processes. Within the spleen we find three macrophage “races”, distributed along the three splenic compartments. Thereby, the macrophages in the red pulp control red blood cell recycling when these cells are very deteriorated. Similarly, macrophages in the white pulp recycle lymphocytes or white blood cells. Between the two pulps there is a zone called marginal zone, where blood filtering takes place. Macrophages in the marginal zone are different from those located in the pulps and they are in charge of detecting and eliminating foreign substances carried in the blood.
Apart from its location, macrophage populations can be identified by a series of proteins embedded into their membranes. In this way, using antibodies that specifically recognize certain proteins, we are able to identify different macrophage populations. Therefore, membrane proteins work like fingerprints, unique marks that help distinguish the type of macrophage present in the sample.
Although macrophage diversity is totally accepted, the way these immune cells differentiate has not been yet well established. All macrophages originate from the bone marrow. However, what is the mechanism that controls functional specialization, that is, that a certain macrophage ends up being a marginal zone macrophage and not a red pulp macrophage?
As an initial approach, this study included the use of genetically modified mice not expressing LXR receptor (α and β isoforms). When the spleen of these mice was analysed, no marginal zone macrophages were detected, but those macrophages present in the two pulps showed normal levels. What’s more, the marginal zone itself was absent; it had disappeared from the spleen. Consequently, these animals had an altered immune function and the filtering role of the spleen was also modified. At the sight of these results, it seemed like macrophage full development depended entirely on LXR receptors: when these proteins were absent, macrophages were not formed and the marginal zone became unclear.
In order to confirm that these changes were linked to macrophage differentiation, a bone marrow transplant was performed. As it was mentioned, the bone marrow originates all types of macrophages and also the rest of the blood cells. The mouse receiving the bone marrow was previously irradiated, so that all its cells from the immune system could disappear. In this way, he would have a totally renewed immune system proceeding from the donor’s bone marrow.
If LXR-deficient transgenic mice received the bone marrow from control mice (those with normal LXR receptors), macrophages from the marginal zone were formed again, as they came from the “normal” bone marrow. However, if these normal mice were irradiated and received the bone marrow from transgenic mice lacking LXR, the marginal zone was progressively lost (it is not possible to create marginal zone macrophages from a LXR-deficient bone marrow).
To ascertain whether macrophage defect depended on one of the two LXR forms, α or β, studies were carried out with mice lacking one of the two isoforms. LXR-deficient mice presented normal marginal zone macrophages; in fact, their bone marrow was capable of restoring that loss in LXR-deficient mice. Therefore, it was concluded that LXRβ was not required to form these macrophages. On the other hand, LXR-deficient mice showed the same results as the ones in transgenic mice without LXR: there were no macrophages in the marginal zone. Accordingly, development of marginal zone macrophages depends exclusively on LXRα signalling.
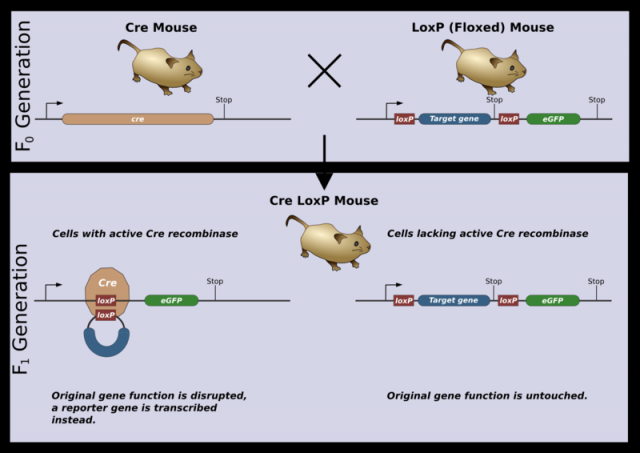
Transgenic mice used in these preliminary experiments lack LXR (or at least one of both isoforms). These receptors are missing in their macrophages, neurons, liver cells, and the rest of their organs and tissues. This generalized absence provokes changes at different levels, which may indirectly affect the formation of these macrophages. In order to discard possible collateral actions, conditional transgenic mice were generated.
Conditional transgenic strategies help researchers to have the expression of a certain protein under control (that is where its name comes from). Conditional transgenic mice produced for this study were only lacking LXRα in haematopoietic cells, blood cell precursors. As macrophages are formed from these cells, they will lack receptors too. These mice are created following this process: mice containing two copies of the LXRα gene are generated, which are flanked by short sequences known as LoxP. These LoxP sequences are recognized by the Cre enzyme, which cuts them out, eliminating the sequence between them (in this case LXRα). Cre enzyme is not naturally present in any of the mice cells.
Mice with the LoxP-flanked LXRα gene are crossed with Cre-expressing mice, which specifically express the enzyme in (precursors of all blood cells, including macrophages). This specificity is achieved by placing the Cre gene behind a promoter that regulates a particular gene of this line. Promoters are DNA sequences that determine where, when and how genes are expressed in the cells. The offspring resulting form such a cross of transgenic mice only presented Cre in haematopoietic cells. Thus, LoxP sequences are only cut in these cells, eliminating LXRα gene. Haematopoietic cells and derivatives (including macrophages) do not have LXRα and the rest of the cells have normal LXRα proteins (as they lack Cre, the sequence between LoxP is not eliminated). These mice showed an identical spleen to that of the initial LXRα-deficient transgenic mice. Macrophage formation in the marginal zone of the spleen depends directly on the expression of LXRα in those very macrophages (or in their precursors).
With the aim of accumulating more evidence concerning this matter, LXRα-deficient mice haematopoietic cells were treated with a specific virus (lentivirus) that enhances LXRα expression. When mice were injected with these LXRα-deficient haematopoietic cells that had been previously treated with the lentivirus, LXRα expression increased and the marginal zone and macrophages form that region were partially restored.
Taken together, these interesting results exemplify the use of protein-deficient mice and shows for the first time the role of nuclear receptors in the generation of specialized macrophage populations.
References
- Noelia A-Gonzalez, Jose A Guillen, Germán Gallardo, Mercedes Diaz, Juan V de la Rosa, Irene Hernandez, Maria Casanova-Acebes, Felix Lopez, Carlos Tabraue, Susana Beceiro, Cynthia Hong, Pedro C Lara, Miguel Andujar, Satoko Arai, Toru Miyazaki, Senlin Li, Angel L Corbi, Peter Tontonoz, Andres Hidalgo & Antonio Castrillo (2013) The nuclear receptor LXRα controls the functional specialization of splenic macrophages Nature Immunology doi: 10.1038/ni.2622 ↩
1 comment
[…] *Imagen de portada: Retrato de familia de las células sanguíneas (Wikimedia Commons). *El artículo completo en inglés está disponible en Mapping Ignorance. […]