Bionanospear, a living nanoprobe with subwavelength resolution
Light waves spread and bend as they pass through an aperture or round the edge of a barrier. This is a well-known phenomenon called diffraction. Diffraction imposes a limit to the size of objects that we can observe sharply using light of a given wavelength, for a given microscope will have a minimum aperture, and this aperture will cause diffraction and, therefore, interferences.
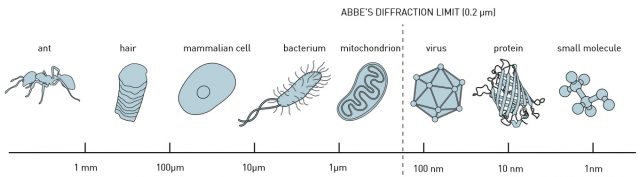
Because of their flexibility and miniaturization, nanoprobes could be considered the default choice for imaging and sensing at the nanoscale, as they would rely on its near-field (close-to-the-sample) capabilities to overcome the diffraction-limit. In general, on the basis of the optical resonances of the nanostructures, nanoprobes can concentrate the illumination light into a confined region, allowing us to probe samples with a nanometer resolution. But are they really the optimal solution for biological-research purposes?
We can see three problems in using standard nanoprobes. The first is that state-of-the-art nanoprobes are typically fabricated with lab-on-a-tip techniques, that is, by integrating nanostructures on the tip of an optical fiber, such as coating with a layer of metal nanofilm, etching with plasmonic nanoantennas, or attaching with a single gold nanorod, semiconductor nanowire, metal nanoparticle, or photonic crystal nanocavity. In other words, to prepare these nanostructures, sophisticated nanofabrication processes and electrochemical reactions are often required.
The second problem for their use in a biological setting is that all this structures have in common their intrinsic lack of biocompatibility as they are made using either a noble metal or semiconductor materials.
And last, but not least, standard nanoprobes are like sharp needles and can easily burst the specimens when interfacing with cells.
Therefore, the ideal nanoprobe would be one that can be made from some biomaterial that we can collect from nature, with some manipulation but no fabrication as such involved, and soft and flexible enough as not to destroy our specimens. Does something like this exist? Well, yes. You have plenty of them yourself: living cells.
Living cells are native optical materials that possess an amazing ability to manipulate light. For instance, algae cells are capable of focusing sunlight to improve the efficiency of phototaxis and photosynthesis, and so behave as microlenses. Additionally, living cells in plant stems function as waveguides that can conduct certain wavelengths of light to the roots. Furthermore, Müller cells in human eyes have the capacity to transmit light from the retina surface to photoreceptors, acting as optical fibers.
So, why not using some biological cells as optical devices? Some pioneering work has been done already: diatom cells, red blood cells, yeast cells, embryonic kidney cells, and Escherichia coli cells have been integrated into functionalized devices, such as force probes, optofluidic microlenses, biological lasers, and biophotonic waveguides.
Now, an international team of researchers including Ikerbasque Research Professor Juan José Sáenz (DIPC), propose 1 a living nanoprobe for near-field probing with subwavelength spatial resolution. They called it a bionanospear.
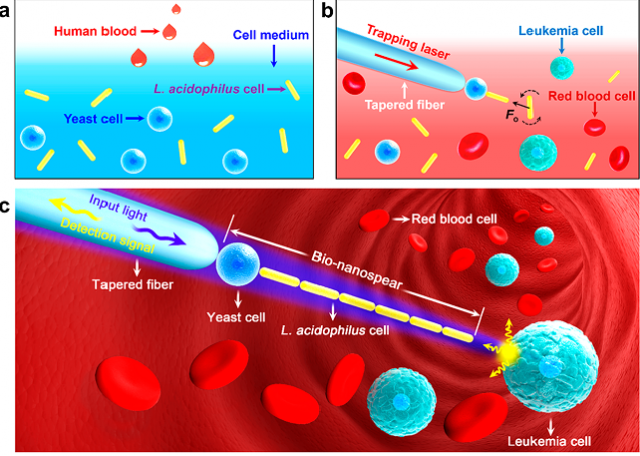
The “handle” of the bionanospear is made of a tapered fiber and the “head” is assembled with a yeast cell and a chain of nanosized L. acidophilus cells. Each part is also a step in focusing light down to the subwavelength region. This means that optical signals are detected in real time for near-field probing at subwavelength resolution.
The bionanospear is flexible and deformable with less chance to puncture and damage the living specimens under measurement, thus it can be used as a noninvasive tool for near-field imaging and sensing of biosamples.The researchers also investigated the light propagation and trapping stability of the bionanospear, building a theoretical model with a finite element method. As a result, the bionanospear can deliver excitation light with a 190 nm spot size to biological samples, with optical signals detected in real time for near-field probing at subwavelength resolution.
The researchers made a successful proof-of-concept experiment measuring localized fluorescence from the surface of single leukemia cells in human blood.
With the advantages of its high resolution and biocompatibility, the bionanospear is likely to find important applications in the fields of biosensing and bioimaging.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
References
- Yuchao Li, Hongbao Xin, Yao Zhang, Hongxiang Lei, Tianhang Zhang, Huapeng Ye, Juan Jose Saenz, Cheng-Wei Qiu, and Baojun Li (2018) ACS Nano 2018 12 (11), 10703-10711 doi: 10.1021/acsnano.8b05235 ↩