Structure matters in the androgen receptor
The study of nuclear receptors includes a great variety of approaches and techniques: from experiments with transgenic mice assessing their roles in the organism, to three-dimensional methods at the atomic level, in which the position of each amino acid and its function are examined. Dr. Estébanez’s research line focuses in the latter feature.
The group studied 1 the three-dimensional structure of the androgen receptor (AR). Androgens are known as male sex hormones. The AR is essential for both male and female physiology, development and metabolism. Mutations in this receptor are associated with diverse pathologies, mainly in masculine individuals. Thus, patients with prostate cancer or androgen insensitivity syndrome show alterations on the amino acidic sequence of their androgen receptors.
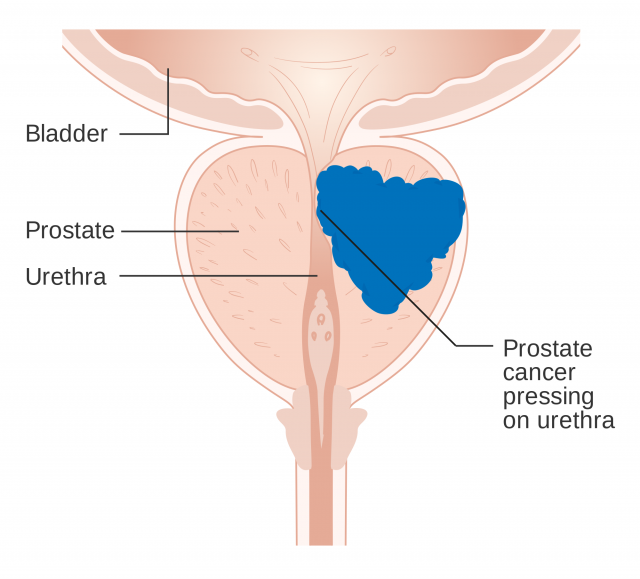
Analysing the 3D crystal structure of AR leads to a better understanding of its role (and, hence, of the role of every nuclear receptor). Besides, it enables researchers to examine the effect of mutations on the receptor functions. Therefore, this represents an essential step towards the comprehension of illnesses derived from AR mutations, and it is also fundamental to the design of drugs that will specifically modify some roles of the receptor without affecting other functions.
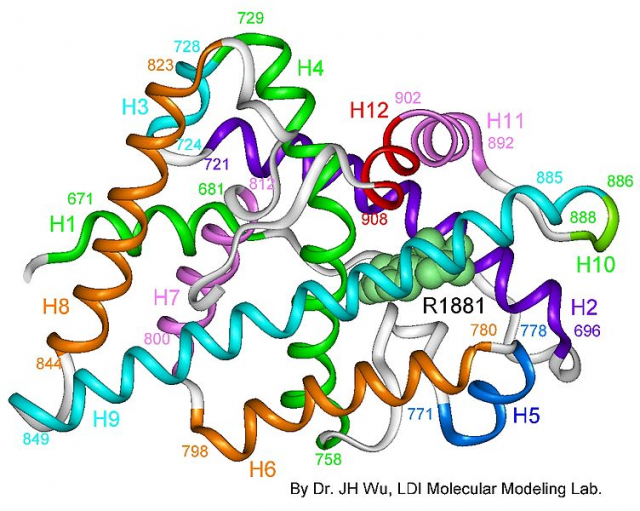
Dr. Estébanez’s group concentrated on the study of androgen receptor ligand binding domain (LBD) structure bound to its natural ligand (dihydrotestosterone) and to a coactivating peptide. This complex was crystallized and analyzed using X-ray diffraction in the ALBA synchrotron, located in the campus of the Autonomous University of Barcelona.
This analysis allowed to elucidate a 2,15-Å 3D crystal structure (0.215 nm or 2,15 · 10-10 m). The distance between the oxygen atom and a hydrogen atom on the water molecule is smaller than 1 Å, so this gives us an idea of the sharp resolution of the method employed in this study. Using an analogy, this would be like looking at a 7-inch Smartphone screen when our body was bigger than the orbit of the Moon. A magnificent example of infinitesimal precision.

This accurate image makes it easier to locate every atom in the folded aminoacidic chain of the receptor. Protein 3D-folding is decisive, because it ultimately determines functionality.
Punctual mutations are genetic modifications that lead to a single change in the aminoacidic sequence. These changes affect protein folding, and, consequently, alter protein function.
The study identified a 1,000 Å2-structure responsible for receptor dimerization. Different mutations associated with prostate cancer or androgen insensitivity syndrome were located in this exact region. Hence, it can be inferred that these mutations modify LBD, affecting dimerization and AR function.
In order to simulate the effect of mutations on the receptor structure, a specific software was used, which proved that many of the changes on the aminoacidic sequence were actually modifying the folding of the protein on that exact region. Most of the mutations inhibited receptor dimerization; one of them (Y764C), however, stabilized the dimer. In order to verify these simulations, some mutations were tested in vivo using two different techniques.
One of them assessed the effect of each mutation on the transcriptional activity of the receptor. For that purpose, luciferase gene was inserted into the cells and was preceded by a promoter responding to androgens. The androgen receptor binds to these elements and stimulates the fluorescent gene production. Fluorescence emission is related to the capacity of the receptor to promote transcription (increase on the fluorescent signal means an increase on transcriptional activity). Mutated receptors were introduced into the cells, each for every mutation previously detected by the bioinformatic study. Nearly all of the selected mutations showed a decrease on the transcriptional activity, or even a loss (fluorescence diminished or disappeared). However, mutation Y764C, which made the dimer more stable, showed higher transcriptional activity (higher fluorescence) than the wild type version of the receptor.
The other technique, known as acceptor photobleaching FRET, can be used to study protein dimerization, which is observed with a fluorescence microscope. These microscopes have 100-nanometre resolutions, being the biggest cell organelles perfectly visible. If protein interaction occurs at the nanometric level… how is it possible to achieve a 100-nanometre resolution increase?
FRET describes energy transfer between two molecules capable of sensing light. Each florescent molecule absorbs light at a particular wavelength and emits light at another wavelength, this is, they “absorb a colour” and “emit another colour”. The FRET method combines two fluorescent molecules (A and B). The cells that are being studied are stimulated with a green lazer, so the A molecule glows green. Precisely, B absorbs green colour and glows red. The glow of B depends on the distance from A. If A and B are separated, only green is detected, as only A is able to glow. If A and B are close, both green and red are detected. This is a very useful and attractive method to test the distance between two proteins, and, therefore, it improves the resolution of the fluorescent microscope, reaching the nanometric scale.
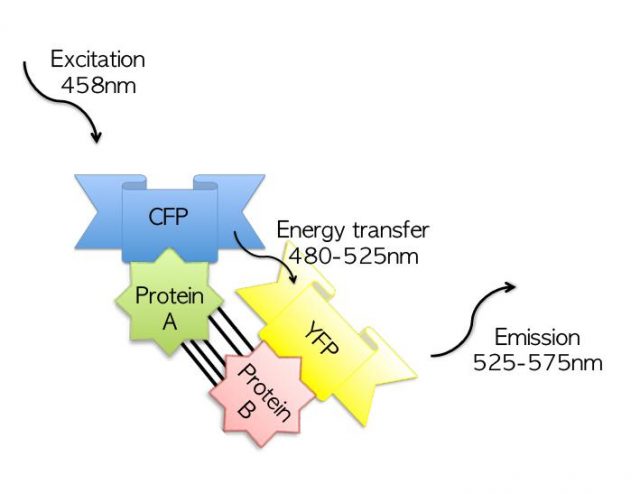
Dr. Estébanez’s lab elaborated a construct formed by AR-LBD bound to the fluorescent molecule A, and another construct where AR-LBD was bound to molecule B. LBDs were mutated and the effect of every single mutation on dimerization was tested. Results matched predictions and they were also consequent with the transcriptional control experiments.
In conclusion, this is a magnificent article that shows the investigation of the structure of a protein at the atomic level and protein-protein interactions, representing a milestone in the study of the dimerization mechanism of nuclear receptors.
References
- Marta Nadal et al (2017) Structure of the homodimeric androgen receptor ligand-binding domain Nature Communications doi: 10.1038/ncomms14388 ↩
2 comments
[…] Read the complete article… […]
[…] *Imagen de portada: Estructura cristalina del receptor de andrógenos humano (Wikimedia Commons). *El artículo completo en inglés está disponible en Mapping Ignorance. […]