Osteoporosis, menopause and the retinoid X receptor
Osteoporosis, menopause and the retinoid X receptor
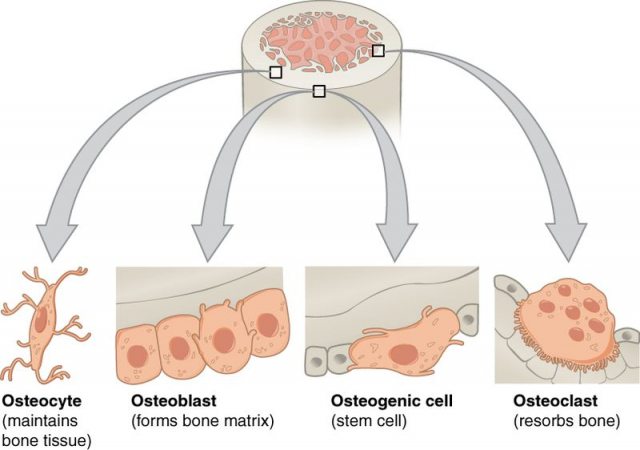
Like the river of Heraclitus, our bones are constantly changing. Although it may look tough and immutable, the bone is a very dynamic tissue that is constantly formed and destroyed, having different appearances during each of the stages of life. Bone destruction or reabsorption is mediated by a particular cellular type known as the osteoclasts (osteo, bone; clasto, break). Osteoclasts originate from haematopoietic stem cells, that is, multi-potent cells that have the ability to renewal themselves and produce blood cells, which is the reason why their name derives from the union of hemato (blood) and poiesis (make).
Both the osteoclast cell number and the control of osteoclast activity are fundamental in order to maintain healthy bones. In fact, deregulation of any of these factors results in an imbalance between bone formation and bone destruction, leading to significant alterations like osteoporosis. This pathology occurs when osteoclast differentiation and activity increases and, subsequently, there is an increment of bone destruction.
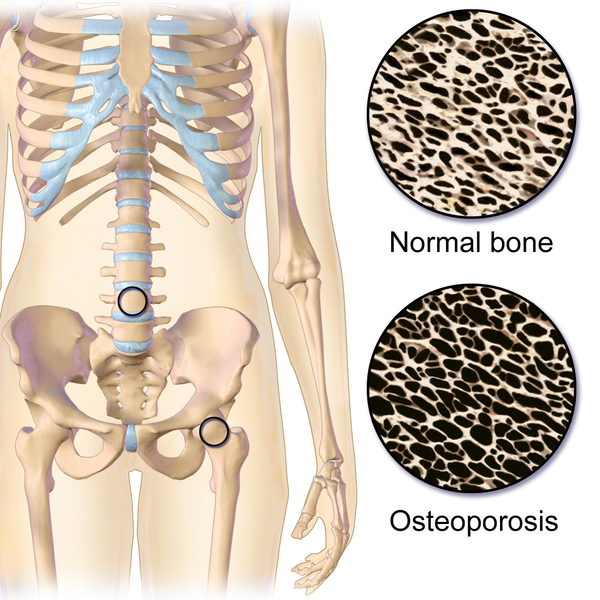
Although osteoporosis is closely related to natural hormonal changes like those happening during menopause, it may be also generated by pathological conditions such as insulin resistance. Currently, drugs used to treat osteoporosis have numerous side effects. For that reason, the study of molecular mechanisms implicated in bone homeostasis (more precisely those involving the control of osteoclast number and activity) may help improve the treatment of bone loss.
Retinoid X receptor (RXR) is a nuclear receptor that controls the expression of a wide variety of genes and some of these genes play a key role in the regulation of glucose metabolism. As insulin resistance contributes to the development of osteoporosis and is directly linked to glucose homeostasis, Dr. Ricote’s group proceeded to investigate the role of RXR in bone homeostasis 1. In order to conditionally suppress RXR activity in haematopoietic cells (and, thus, osteoclasts) transgenic mice were generated. These mice were obtained by crossing mice bearing LoxP-flanked RXR genes with mice containing a gene for Cre recombinase controlled by a certain promoter that is activated in the presence of a substance called pI:pC (polyinosinic-polycytidylic acid). But let’s go step by step.
Cre enzyme recognizes LoxP sequences and cuts whatever there is between them. If there is Cre, the gene flanked by LoxP disappears; if there is not any Cre the gene is expressed normally. In these experiments, Cre is activated solely if mice are injected with pI:pC. Hence, mice are normally developed and after they are born they are injected with pI:pC, which leads to Cre activation in haematopoietic cells. Then Cre eliminates the LoxP-flanked sequences and subsequently haematopoietic cells, including osteoclast precursors, are unable to express RXR.
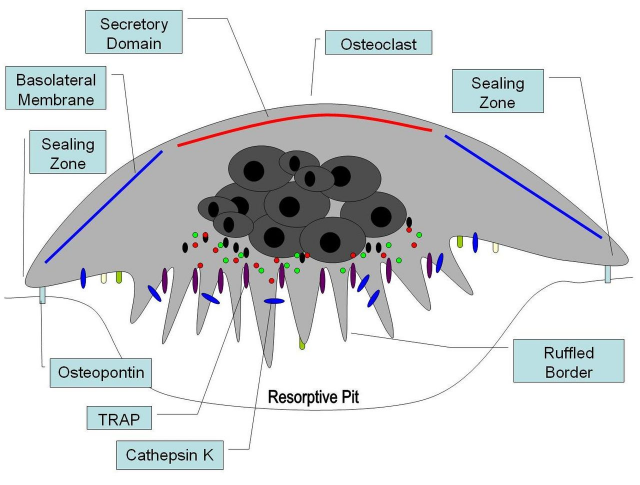
The next question would be: what happens to those mice lacking RXR in their osteoclasts? In the first place, this resulted in formation of giant, nonresorbing osteoclasts and increased bone mass in male mice. But female mice did not go through this physical process. After a thorough microscopic study of the bones, it was confirmed that osteoclasts missing RXR were bigger and were not so tightly attached to the bone, which diminished resorbing activity in comparison to control mice. The authors concluded that RXR played a role in the bone creation/destruction rate, at least in male mice.
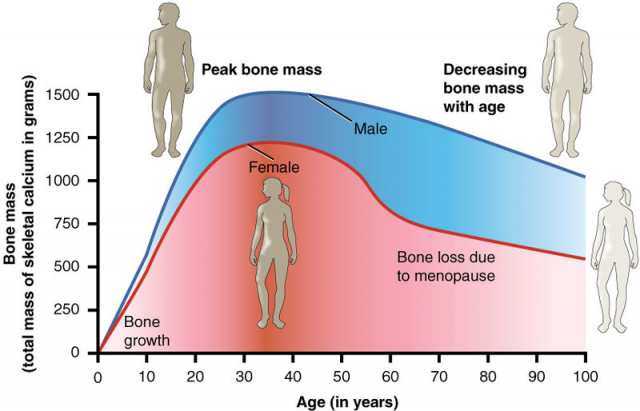
And what about the female? Did the absence of RXR have any physiological repercussions? Not in healthy female mice. However, having in mind the hormonal changes occurred during the menopause, Dr. Ricote’s group extrapolated the study to female mice subjected to an ovariectomy, that is, they had their ovaries removed. Such removal implies an estrogen deficiency that induces menopause symptoms. The same surgery resulted in increased osteoclast activity in wild type female mice (osteoporosis), but this did not happen in osteoclasts without RXR. In other words, RXR induces osteoporosis in menopause mice, for when the receptor is absent both osteoclast activation and bone degradation are lower.
The researchers also used molecular and pharmacological approaches, including cell cultures. In this context, it is possible to differentiate osteoclasts from haematopoietic cells treated with several proteins in the laboratory. These proteins induce the proliferation and further fusion of haematopoietic cells, giving rise to big and multinucleated cells: osteoclasts. Consistent with previous results, osteoclasts lacking RXR were bigger than wild type osteoclasts. Researchers determined that these abnormally giant osteoclasts derived from an aberrant proliferation rate of osteoclast progenitors. As these osteoclasts are bigger, higher bone resorption could be expected. However, the opposite effect was observed: despite their larger size they had a reduced lytic activity.
In order to establish the molecular mechanisms behind these differences, the group studied the expression of different genes that were induced when cells where subjected to the osteoclast proliferation treatment. MAFB was one of the genes that had a reduced expression in cells without RXR. Thus, it was a perfect candidate to explain the changes observed in the osteoclastic differentiation in knockout mice (RXR-deficient).
Schematically, we could establish that:
+RXR → + MAFB → normal proliferation → normal osteoclasts
no RXR → no MAFB → abnormal proliferation → altered osteoclasts
To validate the action of MAFB in osteoclast differentiation, haematopoietic progenitors were infected with modified lentivirus that lead to MAFB overexpression. Results showed that cells lacking RXR but expressing exogenous MAFB had a normal proliferation rate. In conclusion, if osteoclast progenitors do not have RXR they cannot express MAFB and the do not proliferate correctly. However, if MAFB is added interdependently from RXR, osteoclasts recover the standard proliferation rate. Therefore, RXR is proved to be an essential mediator in osteoclast differentiation.
no RXR → no MAFB → normal proliferation → altered osteoclasts
no RXR + MAFB (lentivirus) → normal proliferation → normal osteoclasts
But what is happening to those RXR-containing osteoclasts when MAFB is not present? Using RNA interference techniques (RNAi, small RNA that silences the expression of target genes), it was demonstrated that such cells increased their size and decreased their activity.
+RXR → + MAFB → normal osteoclasts
+RXR no MAFB (RNAi) → altered osteoclasts
Subsequently, molecular RXR activation was assessed in order to verify that MAFB gene was controlled by RXR. All of these compounds induced MAFB as long as RXR was present in the cell. Paradoxically, the authors proved that if MAFB was induced with RXR-activating molecules during the osteoclast differentiation process, this was completely blocked. That is to say, both RXR absence in the beginning of osteoclastic differentiation and further activation once the differentiation process had started had the same effect: a decrease of the osteoclastic activity.
And last, but not least: could these results have a therapeutic application? With the purpose of answering this question mice were treated with a drug that activates RXR (a RXR ligand) commonly known as bexarotene, which is a usual a treatment for skin lymphoma. Bexarotene had no effect on bone density in normal mice. However, when administrated to female mice following ovariectomy (model of menopause) a clear recovery was observed, for these animals presented inferior bone degradation and osteoclastic activity than untreated female mice.
On the whole, based upon the results obtained, it could be concluded that the use of RXR modulating molecules may be useful for the treatment of bone pathologies such as osteoporosis. Given that bexarotene presents some adverse effects, it would be desirable to develop new RXR modulating drugs in order to avoid those negative impacts.
References
- María P. Menéndez-Gutiérrez, Tamás Rőszer, Lucía Fuentes, Vanessa Núñez, Amelia Escolano, Juan Miguel Redondo, Nora De Clerck, Daniel Metzger, Annabel F. Valledor, and Mercedes Ricote (2015) Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling The Journal of Clinical Investigation doi: 10.1172/JCI77186 ↩
2 comments
[…] Read the complete article… […]
[…] Check our latest article in Mapping Ignorance: BPA and pregnancy: diabetes for you and your kids. […]