Catalysis depends on the crystallographic plane of the catalyst
Catalysis depends on the crystallographic plane of the catalyst
A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. As the catalyst itself takes part in the reaction it may undergo a physical change. Hence, if catalysts take the form of nanoparticles, any physical feature of the nanoparticle interacting with the reacting molecules may be relevant for the reaction. One of this features is the orientation of the different crystallographic facets present in a catalytic nanocrystal. Is there a variation of catalysis depending on the crystallographic plane considered?
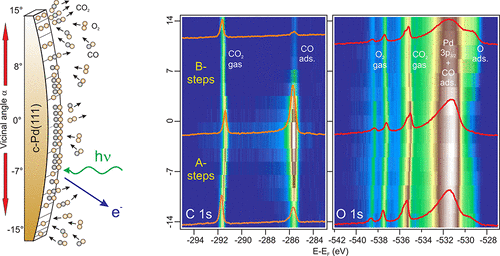
Carbon monoxide (CO) oxidation (2CO + O2 → CO2) on platinum group metal surfaces, such as palladium, is the model heterogeneous gas/surface catalytic reaction. It has been intensively studied during the past decades due to its enormous technological impact. In earlier times, surface science experiments carried out in ultrahigh vacuum played an essential role in the basic understanding of the CO oxidation process, but a much deeper atomic-scale insight is being gained lately through surface sensitive techniques that operate at millibar and bar pressures, such as high-pressure scanning tunneling microscopy, near-ambient pressure X-ray photoemission spectroscopy, infrared reflection absorption spectroscopy, and high-energy surface X-ray diffraction.
All these studies agree on the fundamental picture, namely, the abrupt transition at the “ignition” temperature, from the low-temperature oxidation stage, when CO covers (or “poisons”) the catalytic surface, to the high-temperature activity stage, when the CO-poisoning layer is displaced by chemisorbed oxygen, preceding the build-up, first, of two-dimensional surface oxides and, last, of bulk oxides. Yet new experiments and approaches are needed to properly identify the driving mechanisms and the active sites that trigger the passage to the active stage.
In the case of palladium, the CO oxidation reaction has been investigated on a variety of high and low symmetry crystal surfaces under reaction conditions. This is supposed to help to separately understand the catalytic performance at crystal planes that shape technologically relevant nanoparticles. But, in the end, all facets in nanocrystals coexist in a reduced space, and are assumed to undergo simultaneous structural and non-permanent chemical transformations during catalytic reactions. Therefore, more realistic model systems are needed to fill this gap between individual crystallographic planes and nanoparticles.
Now, a team of researchers, coordinated by Enrique Ortega (CFM, DIPC & UPV/EHU), has built 1 such a model, a cylindrical crystal sample that contains a selected variety of surface planes, which can be exposed to the same reacting conditions, and on which the full analytical power of near-ambient-pressure surface sensitive techniques can be preserved.

The cylindrical surface approach is schematically depicted in Figure 1 for a palladium crystal. The curved direction of the sample spans the complete set of vicinal orientations for the two type of close-packed atomic steps (called A and B) around the (111) symmetry direction. Since the radius of curvature of the sample three orders of magnitude larger than the X-ray light spot, separate crystallographic planes can be sequentially probed by macroscopic sample scanning. In contrast to the full-cylinder approach tested earlier in catalysis studies, this sample design makes it simple to selectively probe all vicinal orientations with a standard synchrotron photon beam.
The researchers use near-ambient pressure X-ray photoemission spectroscopy to identify surface chemical species in stable reaction conditions at fixed temperatures around the ignition point. The result is a consistent description of the surface chemistry during the catalytic oxidation of CO on Pd(111) and its vicinal planes. The strong spatial modulation demonstrates that the reaction is activated in the local plane, with a clear A−B asymmetry. Two parameters appear to be responsible for this behaviour: the α-dependent variation of the CO chemisorption energy and the structural transformation of the surface, likely oxygen-induced faceting, beyond critical vicinal angles.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Frederik Schiller, Max Ilyn, Virginia Pérez-Dieste, Carlos Escudero, Cristián Huck-Iriart, Nerea Ruiz del Arbol, Benjamin Hagman, Lindsay R. Merte, Florian Bertram, Mikhail Shipilin, Sara Blomberg, Johan Gustafson, Edvin Lundgren, and J. Enrique Ortega (2018) Catalytic Oxidation of Carbon Monoxide on a Curved Pd Crystal: Spatial Variation of Active and Poisoning Phases in Stationary Conditions Journal of the American Chemical Society doi: 10.1021/jacs.8b09428 ↩