Cardiac glycosides are a novel family of senolytic compounds
Authors: Pilar Picallos-Rabina and Manuel Collado are researchers at the Laboratory of Stem Cells in Cancer and Aging, Health Research Institute of Santiago de Compostela (IDIS), Santiago de Compostela, Spain

Wouldn´t it be great to have a drug that could kill specifically our old damaged cells allowing us to have a better health at an advanced age? But we are not talking about a dreamed magic immortality pill. Senolytic drugs, compounds that kill senescent cells, are already a reality and represent a new concept that has emerged in very recent years and that promises the possibility of developing therapies for age-related diseases and possibly even for cancer.
Normal cells in our organism have a limited proliferation potential, they can divide and function for a limited number of cell divisions before they cease proliferation and enter a state of permanent cell cycle arrest known as cellular senescence. Cells in senescence are alive and metabolically active but they are uncapable of undergoing further cell divisions. This process was discovered almost 60 years ago when pioneers of the cell culture technique realized that normal primary human cells extracted from tissues and put in plastic dishes in the laboratory could adapt and divide happily for a while before entering this special state known as senescence. This process was suggested to represent cellular aging. Cells in culture were behaving similarly, they thought, to what organisms do during life: they grow and divide before entering an “aging state”. Interestingly, cancer cells never stop proliferating and they would divide in cell culture and in vivo indefinitely.
Later on, it was shown that different aggressions to our cells can also trigger this state of senescence. Damage to our DNA, oxidative stress, activation of oncogenes, etc, can all induce senescence. In this way, damaged cells are restricted in their proliferation, avoiding the emergence of cells that could potentially grow to form a tumor.
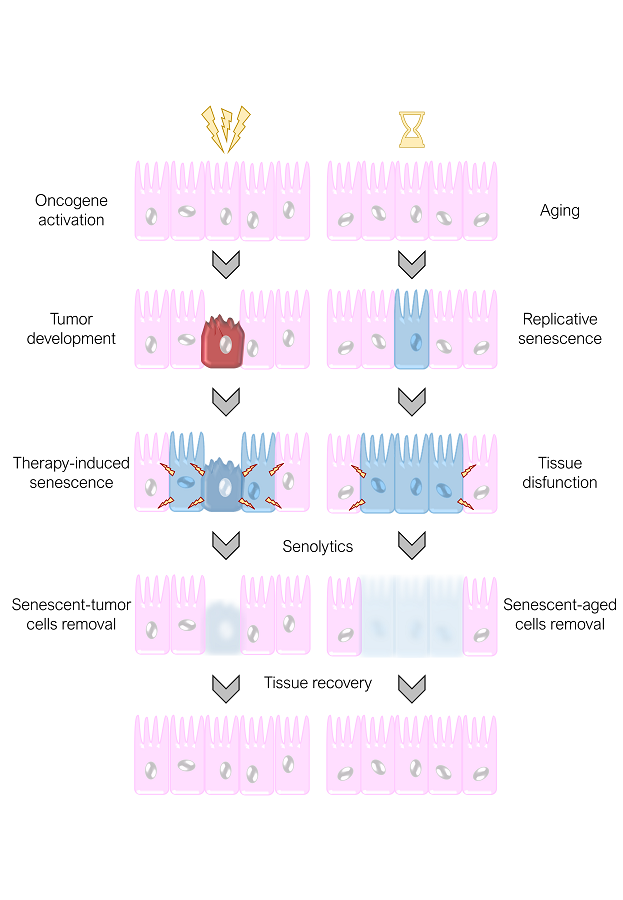
During life, as we age, these senescent cells are generated and accumulate with time contributing to aging and age-related diseases. Too many senescent cells that are not cleared efficiently build up and have detrimental activity due not only to their incapacity to proliferate but also mainly through their secretions. Senescent cells release huge amounts of soluble factors with potentially dangerous activities such as promotion of inflammation, remodeling of the cellular matrix, or induction of proliferation and invasiveness.
Even though cancer cells deactivate the senescence response as part of their tumorigenic conversion, chemotherapy can still induce senescence in tumor cells. These senescent tumor cells might not be eliminated and could represent a risk for patients. Carrying damaged tumor cells around with the potential to re-grow has been proposed to be the basis for tumor relapse, a frequent and lethal event in cancer therapy. Also, senescence induction in tumor, and in normal cells, can have profound effects on neighboring tissue as a result of exposure to the secreted factors released by the senescent cells. Actually, some of the secondary effects produced by chemotherapy in cancer patients seems to be the result of senescence induction in normal cells.
Recently, it was shown that senescent cells can be targeted by cytotoxic compounds with a specific senescent cell killing activity, compounds known as senolytics. These compounds exploit acquired vulnerabilities in senescent cells that make them susceptible for their cell killing activity.
Senolytics can kill senescent cells that accumulate during aging and have been proved to have a positive effect on the healthspan of aged mice and in models of age-related diseases caused by the accumulation of senescent cells. In principle, senolytics could also be used as a means to improve cancer therapy by removing senescent tumor cells produced by chemotherapy, reducing at the same time the secondary effects caused by induction of senescence in normal cells.
To identify novel senolytics we decided to search for this type of compounds in collaboration with Edu Dominguez and Mabel Loza, from BioFarma group at CIMUS (USC) experts on drug discovery. We designed a screening assay based on the human lung cancer cell line A549. We differentially labeled these cells in red, and induced senescence in them, or in green, and left them proliferating. We then mixed both types of cells in the same culture, measured the ratio of green/red cells and treated them with a known senolytic compound, ABT-263 (Navitoclax) or a library of compounds. After this treatment, we measured again the ratio of green/red cells, made some calculations comparing the ratios before and after treatment, and came up with some candidate hits.
We used the Prestwick library of drug repositioning, composed of 1,280 already known and approved drugs. In this way, we could have an idea of the target and pathways involved because the compounds are known, and we could easily translate, in principle, this knowledge into the clinic, since the compounds are already approved for human use.
After screenings and validations, we identified some hits, one of them Proscillaridin A, a Cardiac Glycoside (CG). But this is not the CG that is nowadays used in the clinic, so we tested Ouabain (a typical CG) and Digoxin (more frequently used in the clinic for the treatment of several heart diseases). We checked that the senolytic effect: (1) was a property of all the CGs family members, (2) was observed in tumor and primary human cells, but not in mouse cells (more on this later), was independent of the chemotherapeutic reagent used to trigger senescence, (4) or of the type of senescence inducer. More importantly, it was reproduced by other laboratories. Our collaborators at IMDEA Food in Madrid, Pablo J Fernández and Maria Ikonomopoulou, had been searching for senolytic compounds using different systems (plant extracts and venoms). They told us they had some hits and when we checked, bingo, they were all CGs.
We then wanted to figure out the molecular details of this senolytic activity of CGs. For this, we reasoned that the known target of therapeutic activity of CGs could also be the one responsible for the senolytic activity. CGs exert their therapeutic activity in cardiac diseases by binding and inhibiting the Na+/K+ ATPase pump. This enzyme resides in the membrane of the cell and it pumps out Na+ and pumps in K+. Mice (and rodents in general) express a Na+/K+ ATPase pump that is resistant to CGs. They evolved to express a mutant version of the Na+/K+ ATPase pump because they feed on the plants that produce these compounds, such as plants from the Digitalis genus, commonly known as foxgloves. So we managed to overexpress the mouse version of the Na+/K+ ATPase pump on human cells and checked their survival capacity when exposed to Digoxin, and found that they were more protected. Senescent human cells are killed by CGs because they show an imbalanced electrochemical gradient that make them more sensitive to these compounds.
Finally, we wanted to test the senolytic activity of CGs on in vivo models. First, we checked the effect of combining Digoxin as a senolytic together with chemotherapeutic agents given in the clinic to cancer patients and that we know can induce senescence in the tumor cells. In these experiments we observed that the combination was very effective controlling and reducing the tumor, confirming our hypothesis. But we also tested if these compounds could serve as potential therapeutic agents on a lethal age-related disease, lung fibrosis. We induced fibrosis in mice and treated them with Digoxin. The lungs of Digoxin-treated animals showed a fantastic recovery and were almost free of the fibrotic response.
In summary, we have identified a new family of broad spectrum senolytic compounds that can kill normal and tumor senescent cells and we have proved that the use of these compounds for age-related diseases or for cancer in combination with chemotherapeutic agents inducing senescence, holds the promise of novel, more effective and less toxic therapeutic interventions.
References:
The original paper describing these findings:
Triana-Martínez F, Picallos-Rabina P, Da Silva-Álvarez S, Pietrocola F, Llanos S, Rodilla V, Soprano E, Pedrosa P, Ferreirós A, Barradas M, Hernández-González F, Lalinde M, Prats N, Bernadó C, González P, Gómez M, Ikonomopoulou MP, Fernández-Marcos PJ, García-Caballero T, Del Pino P, Arribas J, Vidal A, González-Barcia M, Serrano M, Loza MI, Domínguez E, Collado M. (2019) Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat Commun. doi: 10.1038/s41467-019-12888-x.
More on senolytics:
Childs BG, Gluscevic M, Baker DJ, Laberge RM, Marquess D, Dananberg J, van Deursen JM. (2017) Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. doi: 10.1038/nrd.2017.116.
More on cellular senescence:
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, Gil J, Hara E, Krizhanovsky V, Jurk D, Maier AB, Narita M, Niedernhofer L, Passos JF, Robbins PD, Schmitt CA, Sedivy J, Vougas K, von Zglinicki T, Zhou D, Serrano M, Demaria M. (2019) Cellular Senescence: Defining a Path Forward. Cell. doi: 10.1016/j.cell.2019.10.005.