Leaf-based microreactors for organic synthesis
Author: Daniel González-Muñoz is a predoctoral researcher in photocatalytic processes at Universidad Autónoma de Madrid.
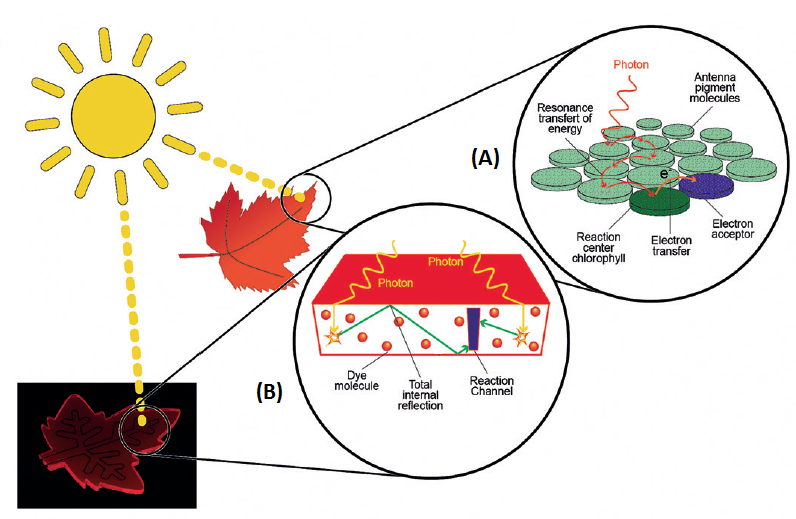
Environmental problems and energy resources make it vitally important for the chemical industry to develop more sustainable and less polluting processes. Therefore, using solar energy is one of the most promising alternatives. If we take a look at Nature’s energetic profile, plants are able to use solar energy to convert it into chemical energy and thus be able to carry out chemical reactions in the photosynthesis process. Plants have a series of antenna molecules in their leaves that are capable of absorbing sunlight and deploying that energy in different chemical reactions and thus make their own food (Figure 1A) 1. It is possible to imitate this process artificially thanks to the field of photocatalysis, which uses molecules capable of absorbing light (photocatalysts) and use it to develop chemical reactions.
Although this field has grown tremendously over the last decade thanks to the development of light-emitting diodes (LEDs), its application using sunlight is very limited. Broad spectral distribution, fluctuations in solar irradiance and the diluted energy content are some of the drawbacks hindering its growth. Nevertheless, these limitations can be overcome thanks to the microreactor design based on the luminescent solar concentrator concept. Luminescent solar concentrator-photomicroreactors (LSC-PM) are slabs of luminophore-doped polymeric materials that harvest, down-convert, and concentrate solar photons (Figure 1B). The LSC-PM feature microchannels through which the chemical reaction that we want to carry out flows (Figure 1B, purple). In photocatalysis, the catalyst can only absorb photons irradiated at a specific wavelength, and therefore cannot take advantage of the rest of photons of different wavelengths irradiated by the sun. However, LSC-PM are doped with compounds that act as antennae and are capable of absorbing photons of the other wavelengths and emitting them at the optimum wavelength for the photocatalyst, mimicking what leaves do for plants.
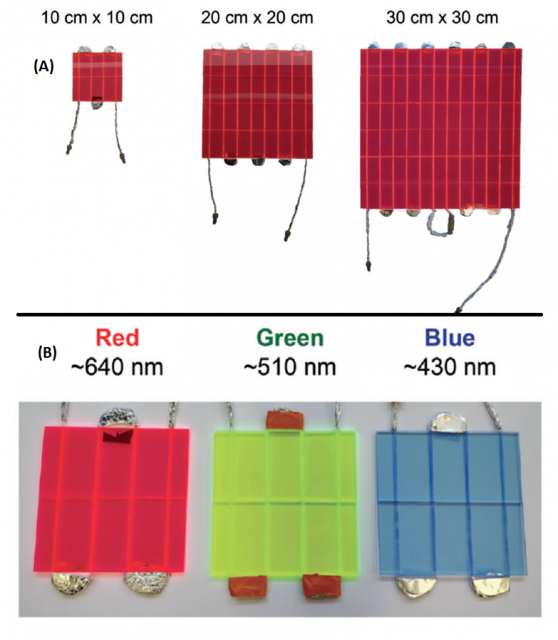
These LSC-PM are made of perfluoroalkoxy alkane (PFA) capillaries embedded in poly(methyl methacrylate) (PMMA) slabs. They are commercially available and easily scalable to different sizes (10×10, 20×20, and 30×30 cm2, from 0.18 to 1.59 mL; Figure 2A). Since each chemical reaction may require a specific photocatalyst, which absorbs at a certain wavelength, it is necessary for the LSC-PM to be doped with antenna molecules that emit in the absorption range of the photocatalyst. For this reason, depending on the photocatalyst of the reaction, an LSC-PM of a certain color shall be used (Figure 2B). For example, the green LSC-PM is doped with an organic fluorescent dye (antenna molecule) called DFSB-K160. This dye emits at 530 nm, which is the wavelength at which photocatalysts such as Rose Bengal or Eosin Y absorb. Therefore, photocatalysts will not only absorb photons that match their wavelength, but also emitted photons by the antenna molecules, which leads to an increase in the photon flux, greater efficiency and superior reaction kinetics.
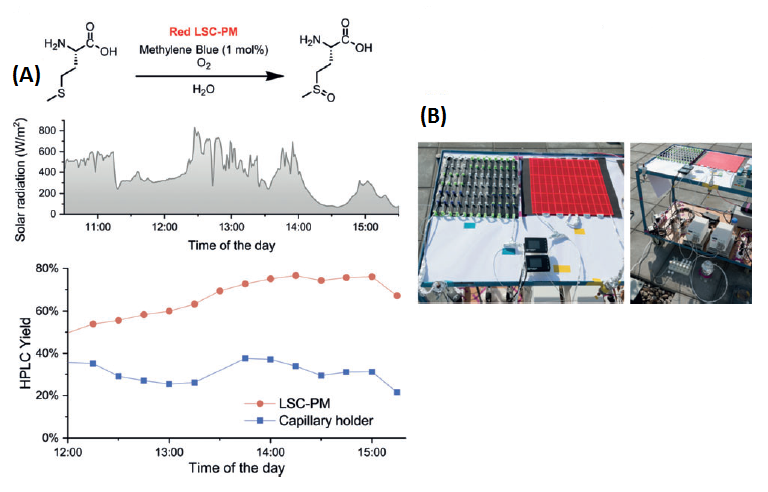
Now that we know how an LSC-PM works, we may wonder which chemical reactions can be carried out, and how conventional synthesis methods may be improved. For example, the red LSC-PM can be used, in combination with Methylene Blue as a photocatalyst, for the oxidation of (L)-methionine (an important amino acid). In addition, the green LSC-PM, combined with Rose Bengal, can perform the oxidation of -terpinene (important for the aroma in perfume and food industry), and the blue LSC-PM, using Ru(bpy)3Cl2, carries out the arylation of morpholine.
As a model example, Figure 3 displays an analysis of how LSC-PM improve the photooxidation of (L)-methionine. This reaction is carried out using the red LSC-PM and using Methylene Blue as a photocatalyst. In Figure 3A we can see the difference between carrying out the reaction in an LSC-PM and in a capillary, that is, without using antenna molecules. The reaction yield is higher when using LSC-PM because of their harvesting ability, which improves the photon flux. In Figure 3B we can see a couple of photographs depicting how the experiment is carried out on the roof of a building.
These experiments demonstrate the efficiency of LSC-PM to carry out chemical reactions with sunlight, but what would happen if the day is cloudy?
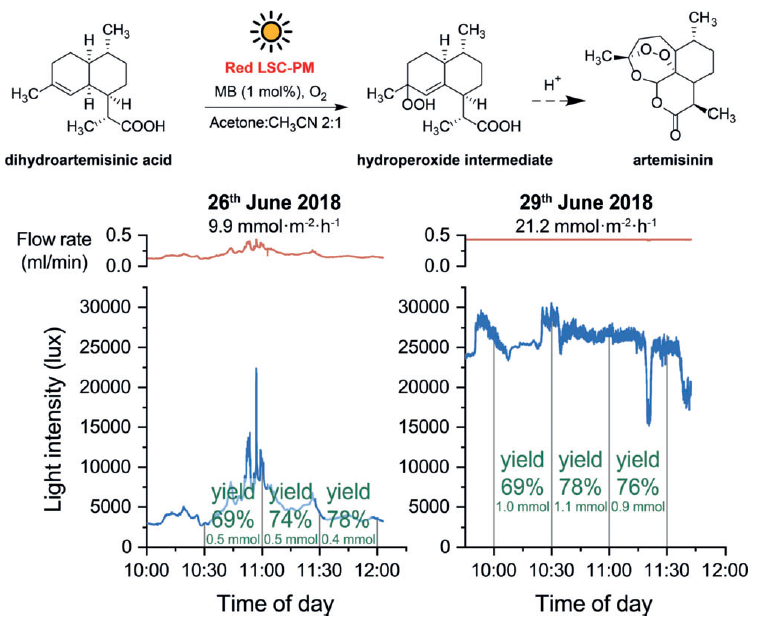
In order to answer this question, a realistic application focused on the synthesis of a drug – artemisinin – was used. This drug and its derivatives are the most effective drugs against malaria, and their yearly production remains insufficient and cannot meet worldwide demand. The crucial step of this process involves the photooxygenation of dihydroartemisinic acid to its corresponding endoperoxide, which yields artemisinin following the acid-catalyzed Hock cleavage (Figure 4, top). The reaction was performed on two different days: cloudy (Figure 4, left) and sunny (Figure 4, right). As you can see in Figure 4, the reaction yield is the same on both days, while productivity does differ. On a sunny day the production is more than twice as high (21.2 mmol·m-2·h-1 vs 9.9 mmol·m-2·h-1) because of the higher irradiance.
To explain these results, two concepts must be taken into account. First, the reaction works on a cloudy day because LSC-PM are not only able to concentrate the direct solar photons, but also those diffused by clouds. On a cloudy day the light intensity is lower, so productivity decreases compared to the sunny day. The other concept to keep in mind is that the pumping system can be adjusted to the light intensity. That is, if the light intensity is very high, the residence time of the reaction in the LSC-PM will be lower. When the light intensity is lower, the residence time will increase. Accordingly, the same yields are obtained regardless of light intensity 2.
This article illustrates that it is possible to develop chemical reactions of interest in organic synthesis with sunlight. Thanks to the LSC-PM, efficiency can be increased dramatically. Furthermore, LSC-PM provide very low luminophore degradation (up to 3% decrease in absorbance over 2 years) and are compatible with a great variety of luminophore dopants.
References
- Dario Cambié, Fang Zhao, Volker Hessel, Michael G. Debije, Timothy Nöel (2017) A Leaf-Inspired Luminescent Solar Concentrator for Energy-Efficient Continuous-Flow Photochemistry. Angew. Chem., Int.Ed., 56, 1050-1054. doi: 10.1002/anie.201611101. ↩
- Dario Cambié, Jeroen Dobbelaar, Paola Riente, Jochen Vanderspikken, Chong Shen, Peter H. Seeberger, Kerry Gilmore, Michael G. Debije, Timothy Nöel (2019) Energy-Efficient Solar Photochemistry with Luminescent Solar Concentrator Based Photomicroreactors. Angew. Chem., Int.Ed., 58, 2-7. doi: 10.1002/anie.201908553. ↩
2 comments
[…] Oso logika erraza da. Landareek eguzki energia baliatzen badute konposatu organikoak sintetizatzeko, zergatik guk ez? Sinplea da ideia, martxan jartzea ez horrenbeste. Aurrerapausuak badaude ere: Daniel González-Muñozen Leaf-based microreactors for organic synthesis […]
[…] La lógica es muy simple. Si las plantas usan energía solar para sintetizar compuestos orgánicos, ¿por qué no hacerlo nosotros? La idea es simple ponerla en práctica no tanto, aunque avances haberlos, haylos: Daniel González-Muñoz en Leaf-based microreactors for […]