How quartz dissolves, bond-by-bond
A silicate is any of a group of substances containing negative ions composed of silicon and oxygen. With this definition it is no surprise that silicates are a huge group. Natural silicates form the mayor component of most rocks, the bulk (90 %) of the Earth´s outer crust and one third of all minerals.
The basic structural unit of a silicate is the tetrahedral SiO4 group. This may occur as a simple discrete SiO44- anion, as in orthosilicates, or as larger silicate species composed of SiO4 tethraedra linked by sharing oxygen atoms as in pyrosilicates. Silicate minerals are classified on a structural basis according to how tethraedra are linked together. Among silicates, quartz is one that seems to escape the definition if we use its simplified formula, SiO2; but, no, it is a tectosilicate, with each silicon−oxygen tetrahedron of the structure linked to other four tetrahedral units, forming a three-dimensional network.
The wide use of silicates in the production of semiconductors for technological applications, insulation materials for the construction industry, or chemical catalysts for different applications, has fostered their study. Of particular interest is the process of silicate dissolution, due to all the previous applications, of course, but also because its importance to understand geochemical and ecological processes, from erosion to explaining their presence in water reservoirs or their role in soil stabilization, etc.
A case in point is the study of quartz dissolution, one of the most abundant minerals in the crust and one having the simpler formula. Actually, the dissolution process can be described by a, also very simple, chemical reaction:
SiO2 + 2H2O → 4 H+ + SiO44-
Quartz dissolution has been extensively studied from the experimental point of view. The variation of dissolution rates with the main variables, namely, pH and temperature, and with the difference in Gibbs free energy (ΔG) between the solid and the solution has been widely studied. Therefore, we know quite a bit about quartz dissolution. We know, for example, that the dissolution rate of quartz is low compared to other minerals due to the high activation energy of the Si−O−Si bond hydrolysis reaction; or that the dissolution rate varies with pH following a U-shape curve with the minimum at pH ≃ 4.8,9 and it is enhanced by the presence of certain salts. But we also know that the activation energy is almost constant independently of the analyzed crystalline plane, and this is at odds with how dissolution rate varies.
All the experimental studies stress the importance of microscopic mechanisms as the origin of the macroscopic dissolution dynamics. At this scale, computational ab initio and Monte Carlo methods represent a powerful tool to study the specific reactions involved in dissolution. Ab initio calculations have been carried out in SiO2 clusters to obtain the hydrolysis energy barriers of Si−O−Si bonds for sites with different degrees of connectivity, from 1 to 4, aiming to identify the limiting reactions which determine the dissolution process, in an unsuccessful effort to explain the inconsistency between the dissolution rate and activation energy.
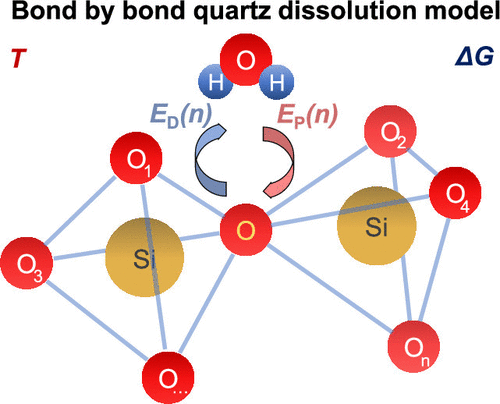
Another approach was, thus, needed. Now, a team of researchers had considered 1 a sequential and independent breakage of bridging oxygen bonds with good results.
They have studied the dissolution using a bond-by-bond reaction process instead of the site-by-site model commonly used in the literature. They have also included the effect of surface stabilization by structural rearrangements and hydrogen bond formation, and taken into account the reversibility of the reactions (something that the standard reaction we wrote above does not). The resulting kinetic Monte Carlo atomistic model shows that dissolution rate and activation energy can be reconciled, while explaining the dissolution rate dependence with ΔG.
This work highlights the importance of building kinetic Monte Carlo models as close as possible to the actual processes to describe macroscopic properties. The methodology could be extended to other minerals.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research papers.
References
- Pablo Martin, Juan J. Gaitero, Jorge S. Dolado, and Hegoi Manzano (2021) New Kinetic Monte Carlo Model to Study the Dissolution of Quartz ACS Earth Space Chem. doi: 10.1021/acsearthspacechem.0c00303 ↩