Tracking C–H bond activation with unprecedented resolution
C-H bond
The emission of methane, a highly potent greenhouse gas, is escalating due to livestock farming and the ongoing thawing of permafrost. Converting methane and longer alkanes into more benign and valuable chemicals presents an opportunity to mitigate these hazards while providing a vast resource for the chemical industry. Nevertheless, the conversion of methane requires the initial disruption of a C-H bond, which is among the most robust chemical connections found in nature.
Four decades ago, the scientific community discovered molecular metal catalysts capable of effortlessly cleaving C-H bonds. Surprisingly, a simple burst of visible light was found to activate the catalyst, resulting in the seemingly magical breaking of sturdy C-H bonds in proximity to it, with minimal energy consumption. Despite the significance of this C-H activation reaction, the precise mechanism by which the catalyst accomplishes this function remained a mystery throughout the years.
Now, in a pair of experiments conducted at the Paul Scherrer Institute in Switzerland, a team of researchers accomplished the meticulous observation of electron exchanges between a rhodium catalyst and an octane C-H group during the bond-breaking process 1. Leveraging the extraordinary capabilities of two of the world’s most powerful sources of X-ray flashes, the SwissFEL X-ray laser and the Swiss Light Source X-ray synchrotron, the entire reaction was traced from its inception to its culmination. The measurements unveiled the catalyst’s prompt activation triggered by light within a remarkable timespan of 400 femtoseconds (0.0000000000004 seconds), leading to the ultimate breaking of the C-H bond after 14 nanoseconds (0.000000014 seconds).
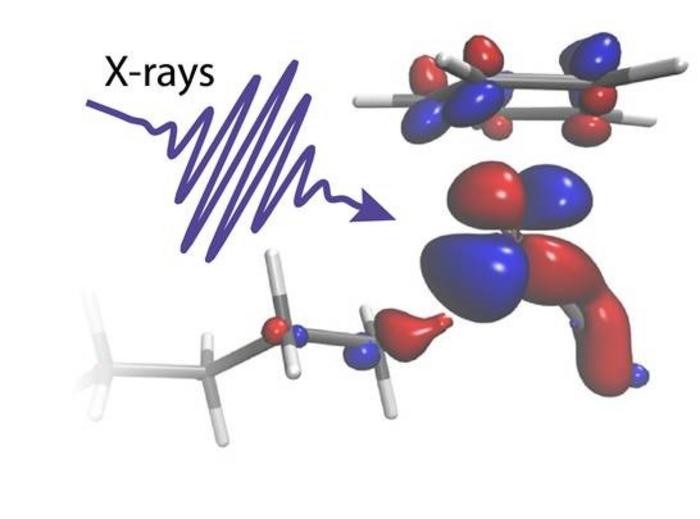
In order to decipher the intricate experimental data, theoretical experts from Uppsala University and Stockholm University collaborated and conducted sophisticated quantum-chemical calculations.
“Through our calculations, we gain a precise understanding of the precise distribution of electronic charge between the metal catalyst and the C-H group, ensuring an optimal balance. We observe that charge transfer from the metal to the C-H bond facilitates the cohesion of the two chemical groups. Conversely, charge transfer in the opposite direction acts as a metaphorical scissor, ultimately causing the separation of the C and H atoms,” explains Ambar Banerjee, a postdoctoral researcher at Uppsala University and the study’s principal theoretician.
This study successfully unravels a perplexing enigma that persisted for four decades: how an activated catalyst can effectively rupture robust C-H bonds through precise fractional electron exchanges, without necessitating extreme temperatures or pressures. Equipped with this newfound knowledge, the researchers now aim to further their understanding of directing electron flow. Their goal is to develop enhanced catalysts for the chemical industry, enabling the transformation of methane and other alkanes into valuable substances, thereby harnessing their potential utility.
More on the sublect:
The anisotropic behaviour of ultrafast electron transfer at the metal/organic interface
The microscopic mechanism behind the vibrational relaxation of adsorbates on metal surfaces
References
- Raphael M. Jay et al. (2023) Tracking C–H activation with orbital resolution Science doi: 10.1126/science.adf80 ↩