The halogen bond – that interaction similar to the hydrogen bond
It seems to be useful to begin this short article with the following quote:
¨with courageous simplification, one might assert that the chemistry of the last century was largely the chemistry of covalent bonding, whereas that of the present century is more likely to be the chemistry of noncovalent binding¨1.
There are different terms for the inter-atomic interactions which play an important role in various chemical processes and reactions and which are not classified as typical covalent bonds. One can mention non-covalent or non-binding interaction, Lewis acid – Lewis base interaction, inter-atomic contact etc. The common feature of a part of them is the meaningful electron charge shift from the Lewis base unit to the Lewis acid one which often leads to further processes, among them to particular chemical reactions.
The hydrogen bond is the most often analyzed non-covalent interaction since it plays a key role in chemical, physical and bio-chemical processes23. One can mention numerous examples such as the role of hydrogen bond in enzymatic catalysis, arrangement of molecules in crystals, crystal engineering, proton transfer reactions, and also its important role in life processes4. This interaction is often designated as A-H…B where the A and B atoms are usually negatively charged while the H-atom is characterized by the positive charge. For example, the O-H…O hydrogen bond which links water molecules is analyzed in numerous studies. The hydrogen bond, because of the opposite charges of the B and H centers being in a contact (the O and H centers in a case of water), is usually classified as the electrostatic in nature interaction. However the mentioned above electron charge transfer processes are very important for the hydrogen bond and this is why also the covalent nature of this interaction is often the subject of disputes and debates. These processes, in a case of the hydrogen bond, often lead to the proton transfer reaction5.
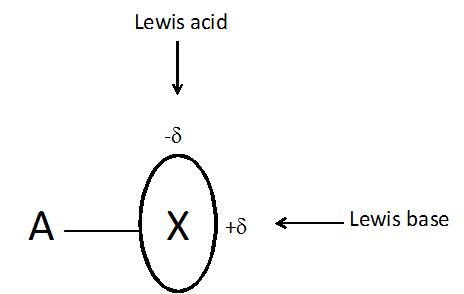
The halogen bond often designated as A-X…B (X is the halogen atom) is another non-covalent interaction which is the subject of numerous analyses, especially in recent years6. This is surprising that this interaction possesses numerous characteristics typical for the hydrogen bond7 where usually negatively charged X-atom, commonly known as an electronegative element, acts as the Lewis acid center interacting with B, the negatively charged Lewis base center! Various concepts were proposed to explain this phenomenon, for example it was stated that the X halogen atom is characterized by the anisotropy of the electron charge distribution what results in the excess of the electron charge (-δ) in the direction perpendicular to the A-X bond and the depletion of this charge (+δ) in the elongation of this bond (Figure 1)8. However it seems that the reason of such anisotropy of the electron charge distribution may be explained in terms of a very simple σ-hole concept9.
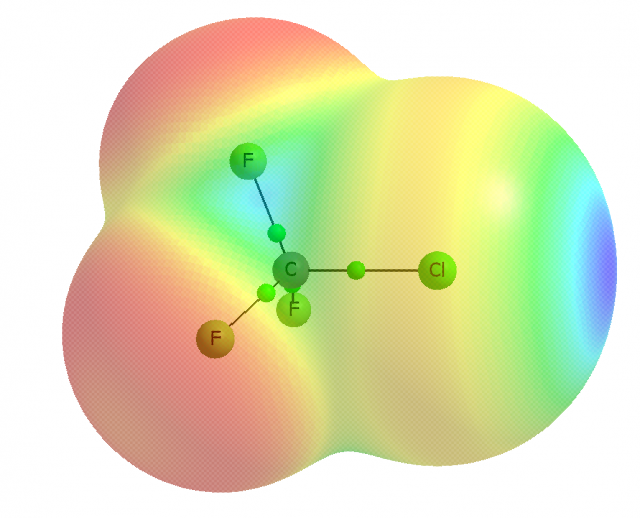
For example, one may consider the s2px2py2pz1 valence electronic structure of the chlorine atom to understand the formation of the σ-hole in CF3Cl. The half-occupied pzorbital interacts with a carbon orbital forming the σ molecular orbital. The electron density of the single occupied pzorbital of chlorine is shifted to the σ-bond region what results in the depletion of this density in the outer lobe of the pzorbital. This depletion is named as the σ-hole and if it is sufficient enough it is characterized by a positive electrostatic potential. This is why the halogen atom, even if it is negatively charged, may be characterized by the area of the positive electrostatic potential and may interact with the electron reach Lewis base centers. An example of the CF3Cl molecule is given in Figure 2. The σ-hole is formed in the elongation of the A-X bond (C-Cl bond in a case of CF3Cl molecule) thus the A-X…B arrangements corresponding to the interactions with the Lewis bases, i.e. halogen bonds, are usually linear or close to the linearity. It was found that the σ-hole is more positive if the more electron attracting is the remainder of the molecule, and the heavier (more polarizable) the halogen (I > Br > Cl > F).
On the other hand the px and py double occupied orbitals of the halogen atom (see Figure 2) form the belt of the negative electrostatic potential around the X-atom, in the direction perpendicular to the A-X bond. One can see that in such a way the halogen atom possesses the dual character since it may act simultaneously with the Lewis bases and with the Lewis acids (see Figure 1).
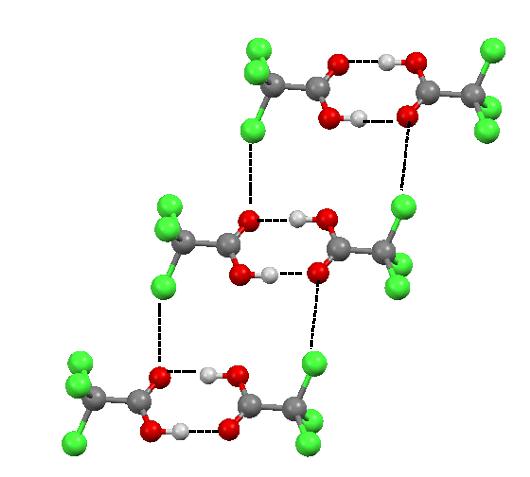
The halogen bonds steered by the distribution of the electrostatic potential in the species being in contact do really exist and they are observed in solids and liquids. For example, in the numerous crystal structures the halogen bonds compete with the more common hydrogen bonds to influence on the arrangement of molecules or ions. Figure 3 presents the fragment of the crystal structure of trichloroacetic acid (TCA). It is commonly known that the TCA molecules are linked by two equivalent O-H…O hydrogen bonds. However there are also the interactions of the CCl3 groups, i.e. C-Cl…O halogen bonds.
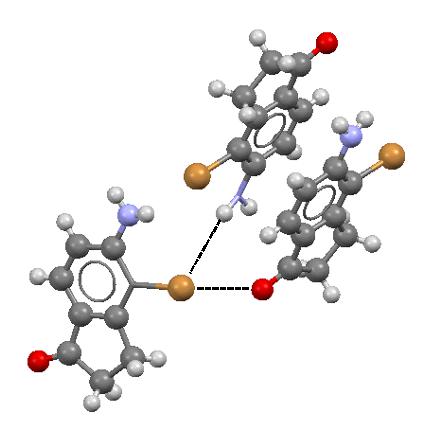
It was pointed out earlier here that the halogen atom, owing to the specific electrostatic potential distribution, may act simultaneously. This σ-hole concept prediction has also the experimental confirmation since numerous crystal structures exist where such a situation is observed. Figure 4 presents an example where the Br-atom has the closest contacts with O and H atoms what corresponds to the C-Br…O halogen bond and the N-H…Br hydrogen bond, respectively.
Thus halogen bonds, similarly as hydrogen bonds, are responsible for the arrangement of molecules in crystals. However there are other numerous studies on halogen bonds as for example in biological systems and drug discovery10. One can also mention that halogen bonds are important in ligand recognition by proteins, and that halogen bonds can be engineered to direct the conformation of DNA junctions.
References
- ¨Binding Mechanisms in Supramolecular Comeplexes¨, Schneider, H.-J. Angew. Chem. Int. Ed., 2009, 48, 3924–3977. ↩
- Hydrogen Bonding in Biological Structures, Jeffrey, G. A.; Saenger, W. Springer-Verlag: Berlin, 1991. ↩
- Hydrogen Bonding – New Insights, Ed. S.J.Grabowski, Springer, 2006. ↩
- ¨Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization¨, Lehn, J.-M. Angew.Chem. Int.Ed.1990, 29, 1304-1319. ↩
- ¨What is the Covalency of Hydrogen Bonding¨, Grabowski, S.J. Chem. Rev. 2011, 111, 2597-2625. ↩
- Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding¨, Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Acc.Chem.Res. 2005, 38, 386-395. ↩
- ¨Hydrogen and halogen bonds are ruled by the same mechanisms¨, Grabowski, S.J. Phys.Chem. Chem. Phys. 2013, 15, 7249-7259. ↩
- Supramolecular chemistry of halogens: complementary features of inorganic (M-X) and organic (C-X’) halogens applied to M-X…X’-C halogen bond formation¨, Zordan, F.; Brammer, L.; Sherwood, P. J.Am.Chem.Soc. 2005, 127, 5979-5989. ↩
- ¨Halogen bonding: an electrostatically-driven highly directional noncovalent interaction¨, Politzer, P.; Murray, J.S.; Clark, T. Phys.Chem. Chem. Phys. 2010, 12, 7748-7757. ↩
- ¨Halogen Versus Hydrogen¨, Metrangolo, P.; Resnati, G. Science2008, 321, 918–919. ↩