The large aromatic nanoring that wasn’t
Aromaticity is a property of cyclic (ring-shaped), planar (flat) structures with π bonds in resonance (those containing delocalized electrons) that gives increased stability compared to other geometric or connective arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily.
An aromatic ring contains a set of covalently bound atoms with specific characteristics:
-
A delocalized conjugated π system, most commonly an arrangement of alternating single and double bonds
-
Coplanar structure, with all the contributing atoms in the same plane
-
Contributing atoms arranged in one or more rings
-
A number of π delocalized electrons that is even, but not a multiple of 4. That is, 4n+2 π-electrons, where n = 0, 1, 2, 3, and so on. This is known as Hückel’s rule.
According to Hückel’s rule, if a molecule has 4n+2 π-electrons, it is aromatic, but if it has 4n π-electrons and has the first three characteristics listed above, the molecule is said to be antiaromatic. The aromatic character of a molecule in the ground state is typically verified experimentally using nuclear magnetic resonance (1H-NMR), as this technique would detect a ring current in aromatic compounds.
Therefore, if the analysis 1H-NMR data coupled with computational calculations points to a molecule being aromatic, surely it is, right? Not that fast. A team of researchers has just proved 1 that is not the case in a six-porphyrin nanoring structure.
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated double bonds. They have the general formula CnHn (when n is an even number) or CnHn+1 (when n is an odd number). Benzene is the smallest neutral annulene that presents π-conjugated aromaticity; other annulenes possessing 4n+2 π electrons are also considered aromatic.
But it is not a general rule, as it may not apply to larger molecules. It is well established that large annulenes suffer out-of-plane distortions and exhibit a poor overlap between π orbitals, thus favoring non-symmetric conformations that are much less aromatic. The larger the annulene, the less aromatic the molecule is expected to be. For this reason, it is difficult to find large aromatic macrocycles. Actually, geometrical constraints are imposed in some large macrocyclic structures with the hope to preserve conjugation, aromaticity, and quantum coherence.
Porphyrin nanorings are very attractive compounds because they offer an end-free p-conjugated system with remarkable properties such as photophysical and guest encapsulating, which might lead to a myriad of applications in the field of single-molecule electronics, serve as light-harvesting antennas, or investigate energy transfer in biomimetic systems, among others.
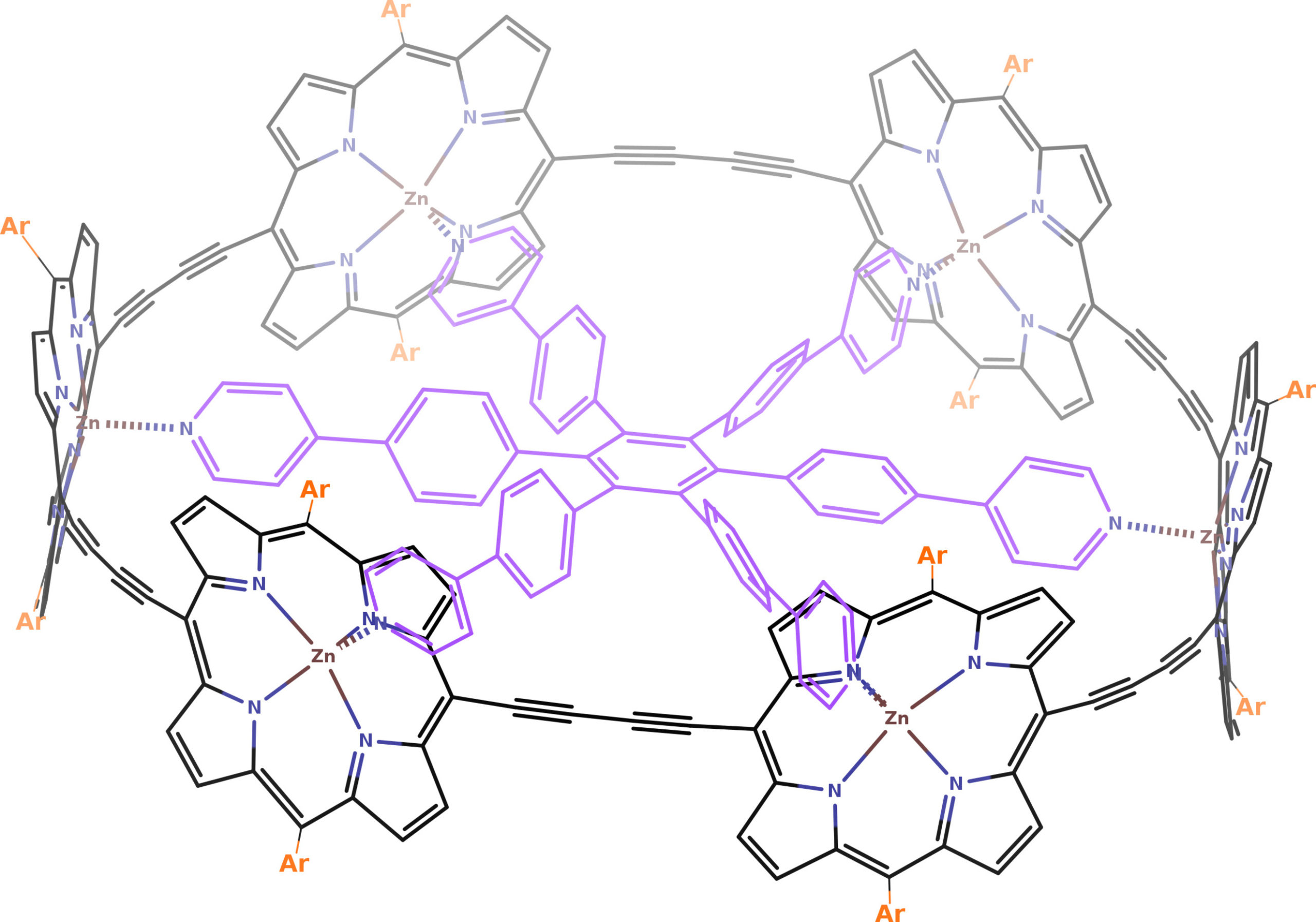
Anderson and co-workers synthesized and analysed the aromaticity of a six-porphyrin nanoring (c-P6・T6) in four different oxidation states (c-P6・T6, c-P6・T64+, c-P6・T66+, and c-P6・T612+), concluding from 1H-NMR and computational analyses that the neutral and the c-P6・T612+ species are nonaromatic, whereas c-P6・T64+ and c-P6・T66+ are, respectively, antiaromatic and aromatic. These molecules were the first of a series of similar large macrocyclic structures exhibiting (anti)aromaticity, allegedly.
Now, a team of researchers demonstrates that the aromaticity of these large macrocycles is questionable. The researchers perform a throughout analysis of these species using several density functional approximations and various tools to analyse the aromaticity, providing compelling evidence that the conclusions are highly sensitive to the level of calculation employed.
The study of electronic structure of nanoring’s four oxidation states results in that the main reason behind the absence of an aromatic ring current in these nanorings is the low delocalization in the transition from the porphyrins to the bridging butadiyne linkers, which disrupts the overall conjugated circuit. Some approximations present such large delocalization errors that they artificially enhance the aromaticity of c-P66+. Hence, 1H-NMR data cannot be used to unequivocally assess the aromaticity of this species.
None of the large nanorings can be considered aromatic, and the quest for large aromatic nanorings should be continued. These results highlight the importance of choosing a suitable computational method to study large conjugated molecules and the appropriate aromaticity descriptors to identify the part of the molecule responsible for the loss of aromaticity.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research papers.
References
- Casademont-Reig, I., Guerrero-Avilés, R., Ramos-Cordoba, E., Torrent-Sucarrat, M. and Matito, E. (2021), How Aromatic Are Molecular Nanorings? The Case of a Six-Porphyrin Nanoring. Angew. Chem. Int. Ed. doi: 10.1002/anie.202108997 ↩