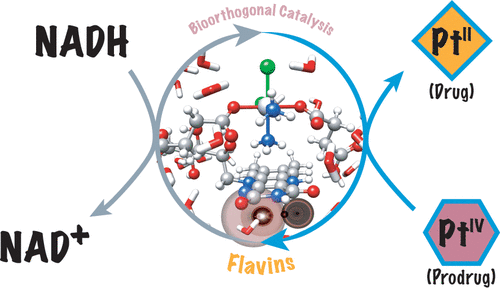
Flavin bioorthogonal photocatalysis mechanism
A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change. As the catalyst itself takes part in the reaction it may undergo a physical change. Metal complexes are typically regarded as catalysts that convert organic substrates into more valuable compounds; however, to date, catalytic transformations of metal complexes are practically unknown and represent a complete new way of thinking in catalysis. Their development can expand the scope of bioorthogonal chemical reactions to inorganic substances and metal-based prodrugs, fostering the creation of new inorganic chemistry toolkits for biology and medicine.
Bioorthogonality, a term coined by Carolyn R. Bertozzi in 2003, refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. Hence, catalytic turnover can boost the efficiency of bioorthogonal chemical reactions, unveiling new strategies for prodrug activation and uncaging of molecular probes.
In 2017, a team of researchers reported a new type of light-driven reaction in which the exogenous biological molecule riboflavin functions as a bioorthogonal photocatalyst and a metal complex acts as an unconventional substrate. This unusual catalyst/substrate pair relies on the photoredox properties of Rf to enable the selective activation of a PtIV prodrug of cisplatin with exceptionally low doses of blue light and induce apoptotic death in PC-3 human prostate cancer cells. Unlike in classic organometallic catalysis, where metals act as catalysts, in this reaction, the metal complex is an unconventional substrate, and the biocompatible riboflavin acts as catalyst.
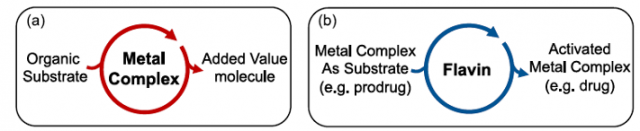
Apart from these pioneering studies on flavins, the enantioselective synthesis of [Ru(bpy)3]2+ via organocatalysis, and the recent aromatic amination of cyclometalated RuII and RhIII octahedral complexes are the three only examples known to date of catalytic reactions that use metal complexes as substrates. This uncharted territory may offer intriguing opportunities to expand synthetic inorganic chemistry and foster new catalysis-based applications for coordination and organometallic compounds.
In order to be able to explore these opportunities it is important to understand how these reactions occur. Now, a new study provides 1 a detailed mechanism through which flavins (photo)catalyze the activation of PtIV complexes.
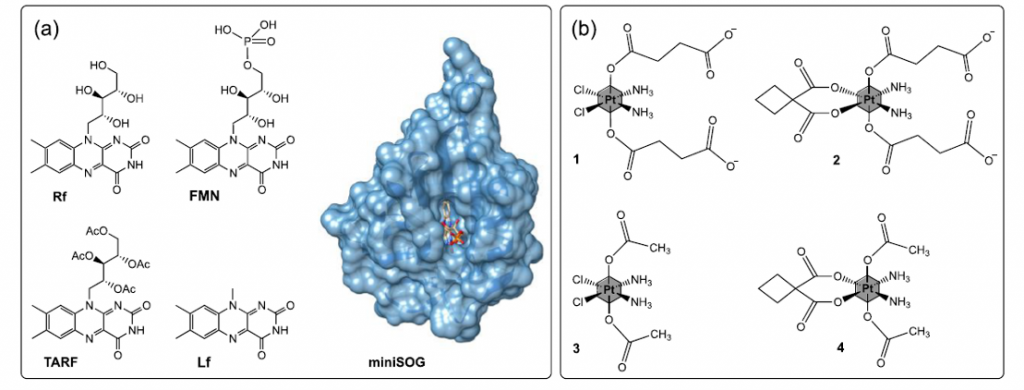
The researchers use four different free flavins (see figure 3), namely, riboflavin (Rf), flavin mononucleotide (FMN), tetra-O-acetyl riboflavin (TARF) and lumiflavin (Lf), and the flavoprotein miniSOG (mini Singlet Oxygen Generator), together with a panel of PtIV substrates conveniently selected. NMR, steady-state, and time-resolved optical spectroscopy are the experimental techniques employed together with density functional theory calculations (DFT).
They find that the activation via reduction of the PtIV complexes may be influenced by H-bonding interactions between the flavin catalyst and the metal substrate and DFT confirms that both the isoalloxazine and ribityl moieties of the flavins determine the catalytic efficiency of the process.
These findings about the redox chemistry of flavins toward transition metals may have potential implications in the biochemistry and in the cell homeostasis metals other than platinum. For example, flavoenzymes such as mercuric reductase regulates Hg resistance in several organisms by promoting the conversion of highly toxic HgII species to less dangerous Hg0.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Juan Gurruchaga-Pereda, Virginia Martínez-Martínez, Elixabete Rezabal, Xabier Lopez, Claudio Garino, Fabrizio Mancin, Aitziber L. Cortajarena, and Luca Salassa (2020) Flavin Bioorthogonal Photocatalysis Toward Platinum Substrates ACS Catalysis doi: 10.1021/acscatal.9b02863 ↩