Nicotine promotes both cancer and metastasis
nicotine
Author: Marta Irigoyen is a postdoctoral researcher at CIC bioGUNE

Tobacco smoke contains a toxic mix of more than 7,000 chemicals. Among them, nicotine is a major cigarette component associated with human tobacco consumption and addiction. Despite not being a carcinogen (an agent that is able to generate cancer), several studies have reported the tumor-promoting effects of nicotine 12. Interestingly, in a recent work, authors go a step further and demonstrate that nicotine is not only involved in tumor progression, but is also able to promote metastasis to the lungs in patients with breast cancer 3. Now, the question is, how nicotine is able to induce tumor metastasis?
The answer can be found in the fact that cigarette smoke has deep effects on immunity and specifically, in neutrophils. Neutrophils are the most abundant leukocyte (50–70%) in human circulation 4, and play a critical role in inflammation and in host defense against microbial infections 5. Although in recent years they have being recognized as a part of the immune reaction to modulate tumor growth and metastatic progression 6, the exact role of neutrophils in tumor progression has been a matter of debate. In the work that I am going to expose, Tyagi and co-workers clarify this question and demonstrate that nicotine activated N2-neutrophils in the pre-metastatic niche (the invironment in a secundary organ that provides favorable conditions for the growth of the metastatic cells) and promotes metastatic colonization of breast cancer cells facilitating tumor progression.
To evaluate the impact of nicotine in distant metastasis, authors injected mouse mammary cancer cell in mice treated with or without of nicotine. Thus, they found that nicotine exposure significantly increased the lung metastatic area and the number of metastatic nodules. To identify which immune cell type(s) in the tumor environment were affected by nicotine, authors examined the composition of different immune cells in primary and metastatic tumors of control and nicotine-treated mice. Among these innate immune cells, neutrophils were the major cell population in metastatic lungs of nicotine-treated mice. These results imply that nicotine may play a critical role in pre-conditioning of the lung immune microenvironment for metastatic colonization. To test this hypothesis, mice were pre-exposed to nicotine or PBS followed by intravenous injection of mouse mammary carcinoma cells. They found that the pre-exposure of nicotine significantly increased the lung metastatic area compared to control mice. To analyze the effect of nicotine abstinence in the context of metastasis, authors pretreated mice with nicotine followed by injecting mamary cancer cells in mice 1 day, 15 days, or 30 days after quitting nicotine treatment. Interestingly, the results indicate that even after quitting nicotine for 30 days, the risk of developing tumor growth and distant metastasis was not prevented.
Contribution of nicotine to metastatic progression
To examine the functional contribution of nicotine-activated neutrophils to metastatic progression, authors performed neutrophil depletion experiment under nicotine-treated or untreated setting by injecting a monoclonal antibody that specifically depletes neutrophils but not monocytes 7. After 24 h of the first nicotine and antibody treatment, mammary cancer cells were injected intravenously into the mice. Remarkably, a significant inhibition in lung metastatic area and in the number of metastatic nodules in the lungs was observed in neutrophil-depleted mice compared to control-treated mice.
To gain further insight into the functional properties of nicotine-treated neutrophils, authors assessed in vitro polarization of human and mouse primary neutrophils using N1-/N2-associated markers. The aim of this experiment was to analyze towards which type of neutrophils they differ after nicotine exposure. Thus, it has been shown that N1-type neutrophils could inhibit the development of cancer whereas N2-type neutrophils could promote the development of cancer. Interestingly, the results showed that nicotine treatment significantly leaded neutrophils toward N2-type. Because nicotine was able to reprogram neutrophils, they next attempted to identify transcription factor(s) that are activated in neutrophils during this process. The analysis revealed that the expression of STAT3 transcription factor (a protein that mediates the expression of a variety of genes and thus plays a key role in many cellular processes) was most significantly increased in N2-neutrophils compared to N1-neutrophils. Importantly, they found a significant reduction of STAT3 in nicotine-activated neutrophils in the presence of a STAT3 inhibitor, and STAT3 silencing led to a significant decrease in the expression of N2 markers. In vivo studies corroborated these results, mice pre-exposed with nicotine showed significantly increased N2-neutrophil accumulation in the lung.
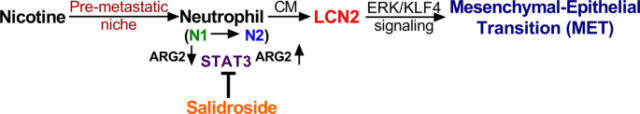
Mesenchymal-to-epithelial transition (MET) play key roles in metastatic colonization 8. Is a reversible biological process that involves the transition from motile, multipolar or spindle-shaped mesenchymal cells to planar layers of polarized cells called epithelia. MET is believed to participate in the establishment and stabilization of distant metastases by allowing cancerous cells to regain epithelial properties and integrate into distant organs. And stromal and immune cells in the tumor microenvironment were shown to have significant impact on this tumor cell plasticity 9. To test the effect of N2-neutrophils on these processes, authors first treated human, mouse primary neutrophils as well as immortalized human neutrophils (HL-60) with or without nicotine and then collected the conditioned medium (CM) to treat mesenchymal-like breast cancer cells. Thus, they found that CM from nicotine-induced N2-neutrophils strongly promoted MET phenotype in cancer cells. Furthermore, they observed a significant increase in tumor cell growth upon treatment with CM derived from nicotine-induced primary human neutrophils compared to the control.
To identify the secretory factor(s) from nicotine-induced N2 neutrophil that mediated MET of cancer cell, authors performed expression profile analysis for human primary neutrophils that were treated with or without nicotine. Remarkably, lipocalin-2 (LCN2) expression was most significantly increased in nicotine-activated N2-neutrophils compared to control cells. LCN2 is a secretory glycoprotein involve in innate immunity and known to be expressed in the neutrophil granules 10. Performing ELISA analysis they found that human primary neutrophils and HL-60 cells secreted significantly higher amount of LCN2 when treated with nicotine compared to the non-treated neutrophils or cancer cells. To further clarify the role of LCN2 in inducing epithelial phenotype, they treated cancer cells with human recombinant LCN2 (hrLCN2). Thus, they found that hrLCN2 at low concentration induced MET phenotype in cancer cells. Furthermore, to examine the direct functional effect of N2-neutrophil secreted LCN2 on promoting MET, authors removed the LCN2 in neutrophils. When tested in vitro, authors observed a significant decrease in MET phenotype and tumor cell growth upon treatment with CM derived from nicotine-induced LCN2 knockout neutrophils (HL-60) compared to the control cells.
The previously mentioned results suggest that nicotine activates N2-neutrophils and supports aggressive cancer cells to metastasize to the lung. Therefore, identifying a drug that specifically block neutrophil polarization would be a promising therapeutic approach to treat breast cancer lung metastasis. Toward this end, authors screened compounds that selectively block N2-neutrophil polarization upon nicotine treatment and they found salidroside as the top candidate. To further assess salidroside suppressive effect on nicotine-activated N2-neutrophil, HL-60 neutrophils were treated with nicotine plus salidroside and examined for N1-/N2-associated markers. Authors found significant decrease in N2-markers in contrast to N1-markers. Similarly, also observed a decrease of active p-STAT3/STAT3 by salidroside under nicotine treatment. Then, they tested in vivo efficacy of salidroside by intravenously injecting breast cancer cells into mice followed by intraperitoneal administration of nicotine with or without salidroside. Remarkably, they found that salidroside significantly decreased nicotine-mediated lung metastatic area and the number of lung metastases nodules. Besides, they showed that nicotine induces infiltration of N2-neutrophils in metastatic tumors and in the premetastatic lungs, and salidroside treatment reversed these neutrophils to N1-neutrophils.
In summary, the risk of tumor growing and distant metastasis apparition was not prevented after quitting nicotine, implying an ongoing risk of metastasis in breast cancer patients with smoking history. Hopefully, results strongly suggest salidroside as a promising therapeutic drug that could be useful in preventing or managing smoking-induced breast cancer lung metastasis.
More on the subject:
Cancer cell clusters to foster metastatic spread
References
- Hawkings BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res 2004; 1027: 48-58. PMID: 15494156 DOI: 10.1016/j.brainres.2004.08.043 ↩
- Mazzaferro S, Bermudez I, Sine SM. α4β2 Nicotinic Acetylcholine Receptors: Relationships between subunit soichiometry and function at the single channel level. J Biol Chem 2017; 292: 2729-2740. PMID: 28031459 DOI: 10.1074/jbc.M116.764183 ↩
- Tyagi A, Sharma S, Wu K, Wu SH, Xing F, Liu Y et al. Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat Commun 2021; 12 :474. PMID: 33473115 DOI: 10.1038/s41467-020-20733-9. ↩
- Mestas J and Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol 2004; 172: 2731-2738. PMID: 14978070 DOI: 10.4049/jimmunol.172.5.2731 ↩
- Kolaczkowska E and Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13: 159-173. PMID: 23435331 DOI: 10.1038/nri3399 ↩
- Coffelt S B, Wellenstein MD and de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016; 16: 431-446. PMID: 27282249 DOI: 10.1038/nrc.2016.52 ↩
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol 2008; 83: 64-70. PMID: 17884993 DOI: 10.1189/jlb.0407247 ↩
- Del Pozo Martin Y, Park D, Ramachandran A, Ombrato L, Calvo F, Chakravarty P et al. Mesenchymal cancer cell-stroma crosstalk promotes niche activation, epithelial reversion, and metastatic colonization. Cell Rep 2015; 13: 2456-2469. PMID: 26670048 DOI: 10.1016/j.celrep.2015.11.025 ↩
- Chen W J, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun 2014; 5: 3472. PMID: 24668028 DOI: 10.1038/ncomms4472 ↩
- Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994; 83: 799-807. PMID: 8298140 ↩