Reducing the energy requirements and carbon footprint of ammonia production
Ammonia (NH3), a ubiquitous compound, was generated in excess of 187 million tons globally in 2020. Roughly 85% is employed in the creation of nitrogenous fertilizers, while the remainder is used for refining petroleum, producing various other chemicals, and manufacturing synthetic fibers like nylon. However, these processes come with a substantial energy expenditure. Presently, the predominant method of producing NH3 involves the Haber-Bosch process, necessitating high temperatures (400-450 °C) and pressures (200 atmospheres) to combine nitrogen and hydrogen. Consequently, researchers are actively pursuing catalysts that can reduce the energy demands of NH3 synthesis and make it more sustainable.
Ruthenium (Ru), a noble metal, has been the leading contender for this purpose due to its remarkable capability to absorb nitrogen at low temperatures. Nonetheless, its exorbitant price has hindered its extensive implementation in large-scale NH3 synthesis. Although cobalt (Co) has been proposed as a more cost-efficient substitute, attaining the same catalytic performance as Ru at low temperatures has proven to be challenging.
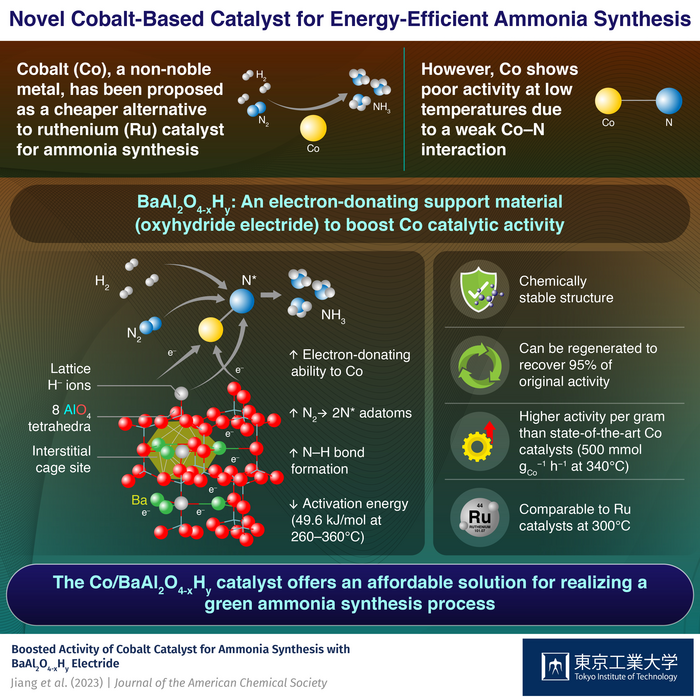
A group of scientists has devised a method 1 to enhance the catalytic effectiveness of Co. In a recent research study, they developed a support material for Co nanoparticles called BaAl2O4-xHy, which is an oxyhydride electride containing barium. This material boosts the catalytic performance of Co to a degree comparable to that of Ru catalysts at low temperatures, while safeguarding H– ions and electrons from the deleterious effects of air and moisture.
The team accomplished this achievement by developing a unique structure for BaAl2O4-xHy, which promotes the dissociation of nitrogen over Co. The material features a 3D network structure with a stuffed tridymite structure where AlO4 tetrahedra are linked, forming cage-like void spaces between the barium ions. These interstitial sites behave like pockets that hold negative charges, allowing the material to donate electrons to Co and facilitate the breakdown of nitrogen molecules into nitrogen adatoms.
To enhance the electron-donating capacity of the material, the researchers replaced the O2– lattice ions with H– ions, which introduced electrons to the interstitial sites. This replacement not only enhanced the electron-donating capability of BaAl2O4 but also facilitated the desired reduction of nitrogen to ammonia.
The Co/BaAl2O4-xHy catalyst achieved a record production rate for Co-based catalysts by promoting both the cleavage of N2 and its subsequent reduction to ammonia, with a yield of over 500 mmol of ammonia per gram of cobalt per hour. Furthermore, the proposed catalyst exhibited an activation energy of just 48.9 kJ/mole, compared to conventional Co catalysts, which typically have activation energies for ammonia synthesis exceeding 100 kJ/mole.
In addition, the stuffed tridymite structure exhibited durability and reusability, as the AlO4-based tetrahedra framework protected the lattice H– ions and electrons from oxidation. The researchers were also able to recover up to 95% of the original activity of the Co/BaAl2O4-xHy catalyst by heating it in hydrogen after being exposed to air.
The Co/BaAl2O4-xHy catalyst has demonstrated great potential for synthesizing ammonia at low temperatures due to its excellent chemical stability, improved catalytic activity, and high reusability. As a result, it offers a new approach to developing highly effective and stable catalysts for green ammonia synthesis without relying on expensive and scarce noble metals like ruthenium. Based on these promising results, the research team believes that their work could significantly contribute to reducing the energy requirements and carbon footprint of ammonia production in the future.
References
- Yihao Jiang, Ryu Takashima, Takuya Nakao, Masayoshi Miyazaki, Yangfan Lu, Masato Sasase, Yasuhiro Niwa, Hitoshi Abe, Masaaki Kitano, and Hideo Hosono (2023) Boosted Activity of Cobalt Catalysts for Ammonia Synthesis with BaAl2O4-xHy Electrides JACS doi: 10.1021/jacs.3c01074 ↩