A new way to rationally design anti-Kasha emitters
Light emission (fluorescence or phosphorescence) in organic molecules, in the vast majority of cases, proceeds from the lowest energy excited state irrespective of the excitation energy used. This is known as the Kasha’s rule, which states that most of the molecules are emissive from the lowest energy, same (ground state) spin multiplicity, S1 excited state. Since it was claimed by Michael Kasha in 1950, few cases with emission from higher excited states have appeared, also known as anti-Kasha emitters.
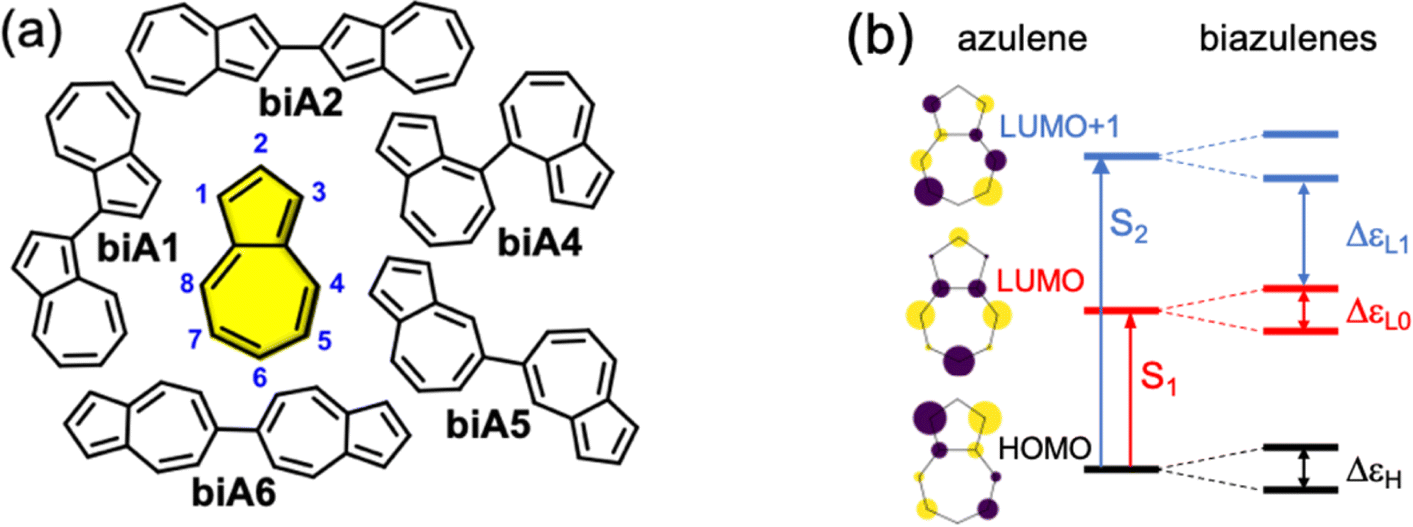
Azulene is a blue crystalline organic compound, C10H8. It contains a five membered rig fused to a seven-membered ring and has aromatic properties. When heated it is converted into its isomer naphtalene. Azulene and its derivatives form a paradigmatic family of anti-Kasha emitters.
Kasha’s rule is based on the fact that closely packed excited states, that is, with small energy separations, always lie with strong vibronic overlaps promoting efficient Sn → S1 non-radiative processes, what is known as internal conversion. Azulene exhibits fluorescent emission from the second excited singlet state (S2), due to, on one hand, the large energy separation with S1 (preventing the efficient internal conversion S2 → S1), and, on the other, the larger oscillator strength of S2 (S2 → S0) compared to S1 (S1 → S0). These two factors combined allow S2-fluorescence to compete with internal conversion.
The violation of the Kasha photoemission rule in organic molecules has intrigued chemists since their discovery, being always of relevance given its connection with unique electronic properties of molecules. However, an understanding of the molecular structure–anti-Kasha property relationship in organic materials has not been well-established, possibly because of the few existing cases available, limiting their prospective exploration and ad hoc design.
Polycyclic conjugated hydrocarbons with non-alternant and antiaromatic topologies have constantly attracted the curiosity of chemists owing to their unique electronic, optical and structural features in comparison with their respective polycyclic aromatic/benzenoid hydrocarbons. Interestingly, azulene is a non-alternant compound, meaning that it has an odd-numbered ring (naphtalene, its alternant isomer, is two fused six-membered rings). Thus, a potential strategy to expand the pool of molecules endowed with anti-Kasha luminescence is via integration of azulene-like fragments in polycyclic conjugated hydrocarbons.
The task to encounter molecules with a fine balance between aromatic/non-alternant composition able to be stable and simultaneously conserve the anti-Kasha anomaly is certainly challenging. A comprehensive understanding of the modes of manipulation of light absorption and emission over broader wavelength ranges of the electromagnetic spectrum, to which anti-Kasha emitters can contribute, is a key aspect for the design of next-generations organic fluorophores able to serve as more versatile and efficient energy conversion substrates.
Now, a team of researchers introduces 1 a novel strategy to design organic emitters from high excited states combining intramolecular J-coupling of anti-Kasha chromophores with the hindering of vibrationally-induced non-radiative decay channels by enforcing molecular rigidity. The researchers apply this new approach to the integration of two antiparallel azulene units bridged with one heptalene all inserted into a polycyclic conjugated hydrocarbon.
With the help of quantum chemistry calculations, the team identifies a suitable polycyclic conjugated hydrocarbon embedding structure and predict its anti-Kasha emission from the third high energy excited singlet state, S3. Steady fluorescence and transient absorption spectroscopy studies corroborate experimentally the photophysical properties in a synthesized chemical derivative with this pre-designed structure.
These results are a proof of concept of a new way to rationally design anti-Kasha emitters and, in general, they encourage the exploration of new strategies of manipulation of light–matter interaction in organic molecules. In-depth multidisciplinary analysis is a unique way for rational design of new chromophores and energy converters in all-carbon-based molecular systems.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Aitor Diaz-Andres, Jose Marín-Beloqui, Junting Wang, Junzhi Liu, Juan Casado and David Casanova (2023) Rational design of anti-Kasha photoemission from a biazulene core embedded in an antiaromatic/aromatic hybrid Chemical Science doi: 10.1039/D3SC00405H ↩