Light-controled deracemization
Just like our hands, certain organic molecules relate to each other like an image and its reflection – a phenomenon that chemists call “chirality” or “handedness”. The two mirror images of the same molecule, namely both enantiomers, often possess different biological properties. This is key, for example, for drug discovery, as many times only one of the structures is relevant.
However, chemical synthesis methods often create a 1:1 mixture of both forms, called racemic mixtures or racemates. Therefore, the selective conversion of these mixtures into one selected form, or deracemization, is of great importance. Now, a team of researchers has developed 1 a novel concept in which this deracemization is enabled by light as an external energy source.
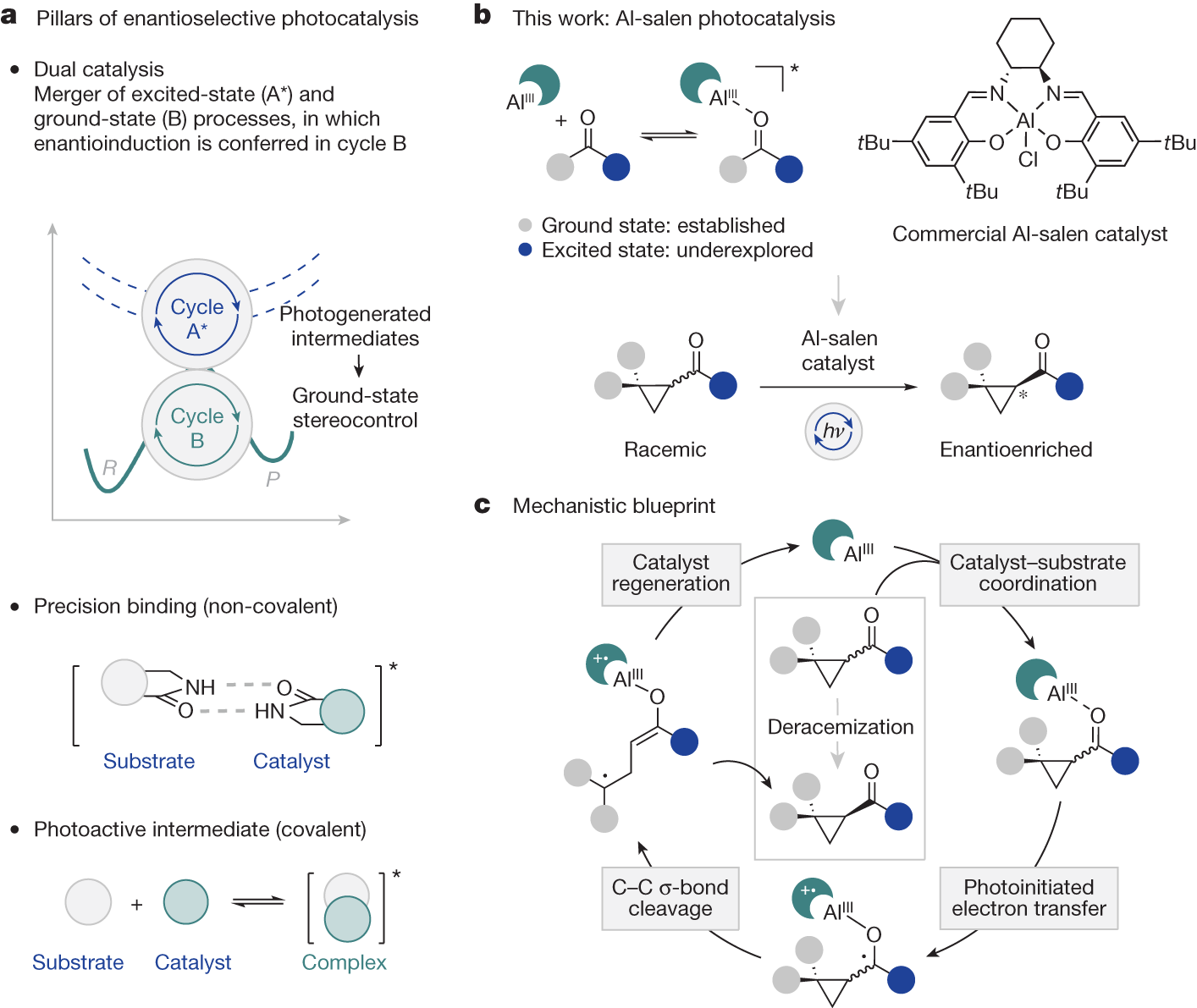
The researchers apply an aluminium complex, that is activated by light, as catalyst to selectively convert a mixture of enantiomers into a single form. The reaction process was investigated experimentally and computationally. The detailed computer-based analyses contributed significantly to the understanding of the underlying dynamics.
Achieving spatial control in light-mediated reactions is one of the main challenges in contemporary organic chemistry. To this end, usually two distinct catalysts are employed in one reaction: a photocatalyst, that initiates the reactivity, operates in concert with a second catalyst that controls the spatial arrangement of the molecules. However, the successful integration of both functions in a single catalyst structure was so far only achieved by the incorporation of tailored recognition motifs in the catalyst and substrate structures.
The new deracemization method implies a catalyst that regulates reactivity and selectivity simultaneously. It binds to simple ketones, a functional group that is prevalent in organic molecules, circumventing the need for tailored components. Furthermore, the catalyst is based on earth-abundant aluminium, which is cheaper that the transition metals that are commonly found in photocatalysts.
It is most impressive the operational simplicity and broad applicability of the new method, as the aluminium complex used is a cheap common catalyst for chemical reactions driven by heat. The light-mediated processes would enable a plethora of new reactivities with great spatial control.
More on enantioselective processes:
A new path to enantioselective substituted pyrrolidines
First nondestructive enantioselective detection technique
References
- Onneken, C., Morack, T., Soika, J. et al. (2023) Light-enabled deracemization of cyclopropanes by Al-salen photocatalysis. Nature doi: 10.1038/s41586-023-06407-8 ↩