Nitric oxide in plants, its mysterious origin revealed
Nitric oxide in plants, its mysterious origin revealed
Nitric oxide (NO) is a gas generated during industrial combustion and by vehicle engines that causes damage to the ozone layer and contributes to acid rain. In this regard, early studies on NO were focused on its nature as a pollutant agent.
In most living organisms, NO is endogenously produced and possess a double face. On one hand, NO is a free radical that tends to react with other molecules, such as reactive oxygen species (ROS). For instance, it can react with superoxide anion (O2–) to form peroxynitrite, a highly reactive oxidant that can react and damage a wide array of molecules including DNA and proteins causing the so-called nitrosative stress. Thus, at high concentrations, NO is considered as cytotoxic. On the other hand, because NO is a small water-soluble gaseous molecule it can pass freely across biological membranes, property that makes that at pico- and nanomolar concentrations NO is an important biological messenger that participates in numerous cellular processes such as in the regulation of gene expression and protein activity. Thus, NO function is concentration-dependent and its action depends on being delivered to a target site at the right dose at the right time.
In mammals, NO participates in many processes, including neural communication and immune responses. Among the diverse functions of NO, one of the first discovered, and the most famous one, is its role as a vasodilator in the cardiovascular system as reported in the early ´80s. Discovery that was awarded with the Nobel Prize in Physiology or Medicine in 1998 (www.nobelprize.org/prizes/medicine/1998/summary/). For instance, through increasing blood flow and oxygen delivery to muscles, NO is useful to improve performance in sport. Indeed, pharmaceuticals such as nitroglycerine, an NO donor, have been used for decades to treat heart conditions. As another example, sildenafil (Viagra) is an inhibitor of a phosphodiesterase-5, which enhances NO-mediated vasodilation. In plants NO plays regulatory roles in virtually every aspect of the plant life going from seed germination, plant development, response to environmental constraints and plant interaction with pathogens and beneficial microorganisms like mycorrhiza.
NO can be synthesized from either oxidized or reduced N-containing precursors. In mammalian cells, NO production is enzymatically generated by the enzyme Nitric Oxide Synthase (NOS), that synthetises NO out of arginine through the oxidation of its guanidine group, also yielding citrulline as a by-product. Three NOS isoforms are present in mammal cells: endothelial, neuronal and inducible NOS. In higher plants, the reduction of nitrite to NO catalysed by nitrate reductase is the most common and accepted pathway for NO synthesis. However, the oxidative origin NO, despite many efforts of the research community, has remained elusive until very recently. In 2003 the existence of a plant NOS was reported in Arabidopsis thaliana 1. However, this protein did not display significant sequence homology with mammalian NOS and was found to lack NOS activity but to act as a GTPase. Thus, the protein described by Guo et al., was renamed as Nitric Oxide Associated 1 (NOA1) 2. Nowadays, it is assumed that land plants do lack NOS enzyme.
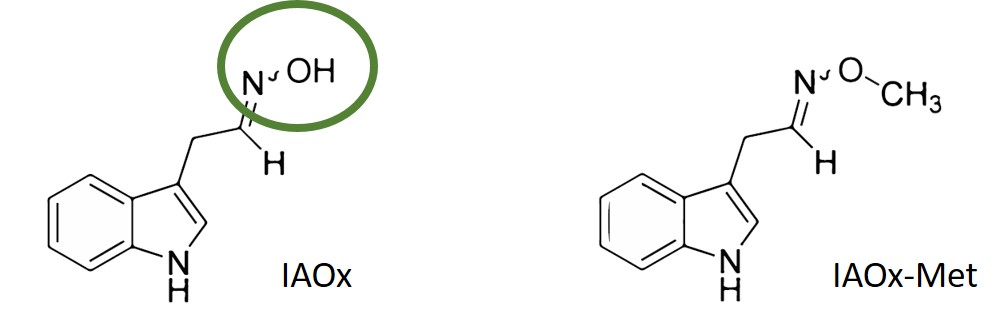
In January 2024 3, the research group of Prof. José Fernando Morán from the Public University of Navarre, in collaboration with researchers of the University of the Basque Country and the University of Tübingen, described the discovery of the long sought mechanism for the oxidative production of NO in plants. López-Gómez et al. described that oximes, a group of organic molecules that contain an N-OH group (Figure 1), are precursors of NO. Most of the work was performed with the oxime indole-3-acetaldoxime (IAOx; Figure 1) that presented an important capacity of NO release. IAOx is a metabolite derived from the amino acid tryptophan and is a precursor of auxin (indole-3-acetic acid), a plant hormone with many different functions, most associated with plant development.
In their work, López-Gómez et al. used two complementary methods for the detection of NO. 1) A fluorescent probe (DAF-FM) that reacts with NO and emits fluorescence, which allows the visualization of NO with a microscope but does not allow a true quantitative NO determination. 2) A chemiluminescence-based NOx analyzer that allows the quantitative and real-time monitoring of NO.
The authors detected that NO generated from IAOx, catalysed by peroxidase (POD) enzymes in a reaction that was boosted in presence of flavins as coenzymes. Oximes were not able to spontaneously yield NO. Importantly, the O-methylation of the IAOX oxime group (IAOX-Met; Figure 1) prevented the generation of NO, thus further demonstrating that the oxidation of the oxime group was the origin of NO. In addition, through density functional theory (DFT)-based modelling the authors provided a reaction mechanism that fully supported the experimental results.
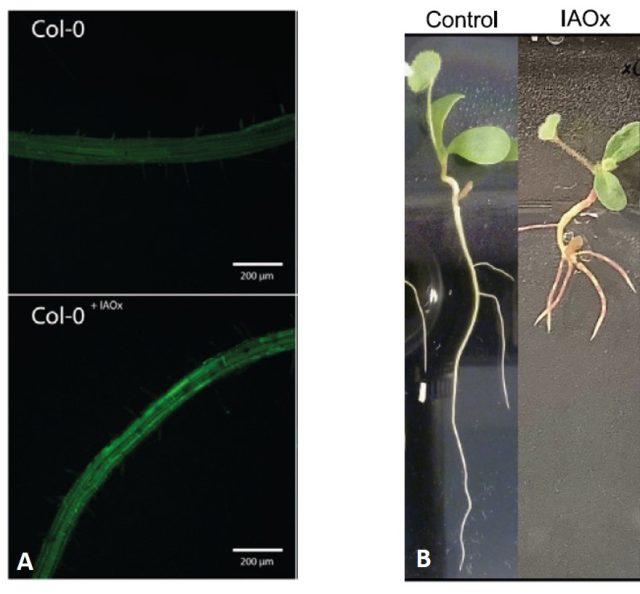
When chemically synthetized IAOx was added to plant extracts or directly to the root it increased the production of NO (Figure 2). In addition, one of the functions of NO relates to the control of root architecture and the application of NO donors is known to stimulate the number of lateral roots, In agreement with oximes function as NO precursors, growing plants in presence of IAOx modified the plant root system architecture, notably increasing the number of lateral roots (Figure 2). Thus, the authors proposed that IAOx may be an additive that can help crops to adapt their root system architecture in order to be more efficient in the exploitation of soil resources, including nutrients and water.
Altogether, the work of Lopez-Gomez et al. evidences that plants produce diverse molecules, such as oximes, that allow the oxidative synthesis of NO. This new route and the properties derived from the oxime molecules can be essential in future studies that aim to exploit NO signalling pathways to improve crops performance, or even to use them in clinica for cardiovascular health.
References
- Guo et al. (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signalling Science doi: 10.1126/science.1086770 ↩
- Zemojtel et al., 2006 Plant nitric oxide synthase: a never-ending story? Trends Plant Sci. doi: 10.1016/j.tplants.2006.09.008 ↩
- López-Gómez et al. (2024) A new oxidative pathway of nitric oxide production from oximes in plants. Molecular Plant doi: 10.1016/j.molp.2023.12.009 ↩