Synthesis of organometallic helicenes by simple combinations
“Helicene” is the name introduced by Newman in 1955, to describe the benzologues of phenanthrene in which the extra ortho-condensed rings give rise to a (regular) cylindrical helix. The pioneer work of Newman in this field cannot be overemphasized; his brillant synthesis and resolution of [6]helicene will remain as a landmark, for it opened the way to the study of a fascinating class of synthetic molecules. 1
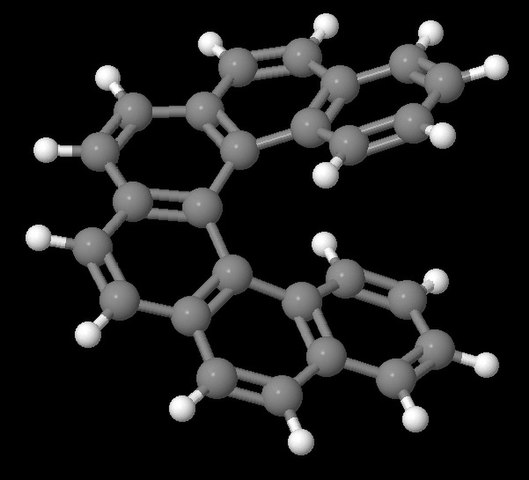
Organometallic helicenes are appealing chiral entities. By combining the chiroptical properties of helicenes with those inherent to the metal centre, they are suitable for the development of multifunctional molecules. Among them, few examples of iridahelicenes appeared in the literature, intended to exploit the outstanding photophysics of iridium(III) bis- or tris cyclometalated complexes, combined with the central chirality imposed by the organometallic core and the helicenic structure.
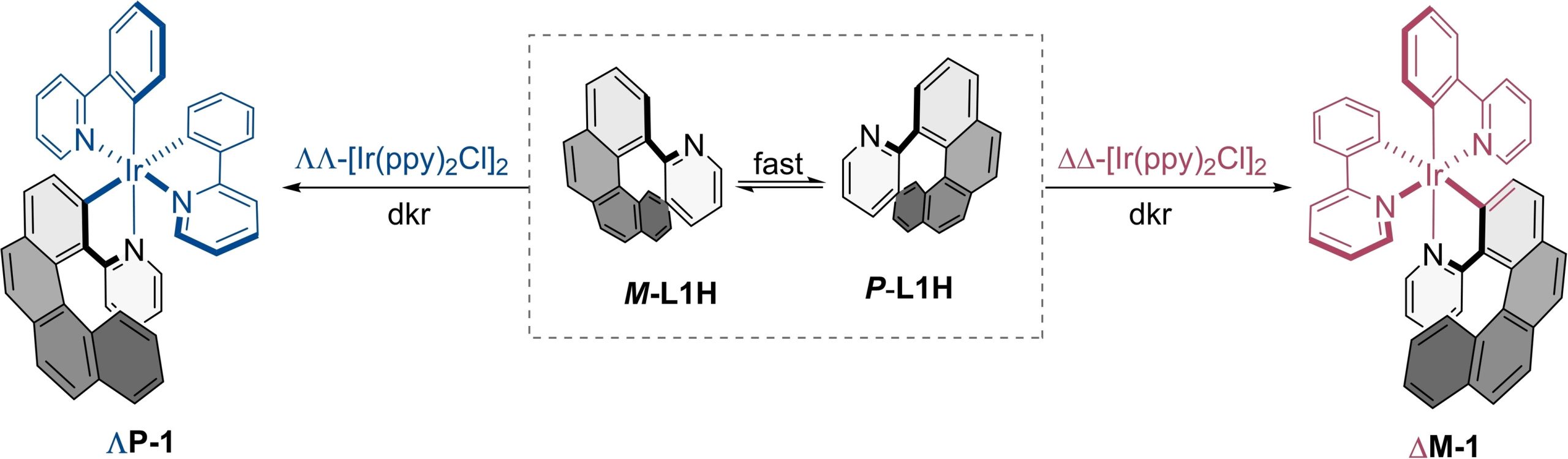
Now, a team of researchers reports 2 the synthesis of a pair of enantiopure [6]-azairidahelicenes incorporating chirality at the metal center and on the helicenic ligand. These two organomatallic helicenes are readily accessible by an effective dynamic kinetic resolution (dkr) of a configurationally labile [4]-helicenic ligand. The two isolated enantiomers exhibit perfect mirrored electronic circular dichroism (ECD) and circularly polarized phosphorescence (CPP) signals.
According to the results, dkr is strongly directed by structural features on the reaction intermediates towards cyclometalation, imposed by the C2-symmetric bis-cyclometalated organometallic core. This type of arrangement is common in many organometallic compounds. Consequently, this synthetic strategy can be extended to obtain not only a variety of chiral [6]-azairidahelicenes but also other metallahelicenes by simple combinations of ready-available chiral-at-metal bis-chelated precursors conformationally stable and epimerizable [4]-helicene derivatives.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Martin, R.H. (1974) The Helicenes. Angew. Chem. Int. Ed. Engl. doi: 10.1002/anie.197406491 ↩
- A. Pazos, C. M. Cruz, J. M. Cuerva, I. Rivilla, F. P. Cossío, Z. Freixa (2024) Angew. Chem. Int. Ed. doi: 10.1002/anie.202406663 ↩