X-rays reveal the true identity of nanoparticles in medicine
X-rays reveal the true identity of nanoparticles in medicine
When you hear the word nanoparticle, it might sound like something futuristic or abstract. In reality, nanoparticles—tiny structures tens of thousands of times smaller than the width of a human hair—are already part of modern medicine. Some are used to deliver drugs more effectively, while others help doctors see inside the body by acting as contrast agents in medical imaging. Their promise is enormous, but so are the challenges.
Which is the real form of a nanoparticle in vivo?
The difficulty lies in the fact that once nanoparticles enter the human body, they rarely remain in the form in which they were designed. A gold nanoparticle, for example, may start out as a neatly engineered sphere with a protective coating. But the moment it encounters blood or tissue fluid, proteins, fats, and sugars in the body begin to stick to its surface. Scientists call this new layer the protein corona. Along with the particle’s original inorganic core and its synthetic coating (often a polymer), this corona defines the “biological identity” of the nanoparticle—that is, how the body actually sees and responds to it.
This is not a trivial matter. The size, shape, and surface chemistry of a nanoparticle strongly affect whether it is taken up by cells, filtered by the kidneys, or flagged by the immune system. Even small changes can determine whether a nanoparticle becomes a powerful therapy or an inert speck cleared away before it can do its job. That is why researchers urgently need tools to look at nanoparticles in their real biological state, not just the pristine form they have in the lab.
Traditional methods give us only part of the picture. Dynamic light scattering, for instance, can tell us the average size of particles in solution, but it struggles when samples contain a mixture of sizes or when the medium is cloudy. Transmission electron microscopy provides beautiful images of nanoparticles, but only after samples have been dried or fixed, which may alter their natural state. Nuclear magnetic resonance and radioactive labeling techniques can follow nanoparticles inside organisms, but they often lack the structural detail scientists need.
This is where X‑ray scattering comes in. X‑rays, unlike visible light, can penetrate tissues and fluids, making them suitable for studying nanoparticles in complex environments. In particular, small‑angle X‑ray scattering (SAXS) is a technique that measures how X‑rays are deflected when they pass through a sample. From the scattering pattern, researchers can deduce the average size and shape of particles in solution. The drawback is that standard SAXS cannot easily tell apart the different layers of a nanoparticle: the dense core, the polymer coating, and the protein corona all blur together.
Overcoming limitations using ASAXS
A recent study 1 has shown how to overcome this limitation using anomalous small‑angle X‑ray scattering (ASAXS). The principle relies on the fact that different elements scatter X‑rays differently, and that their scattering behavior changes sharply when the energy of the X‑rays approaches one of the element’s absorption edges. By tuning the X‑ray energy to these special points, researchers can selectively highlight the contribution of specific elements.
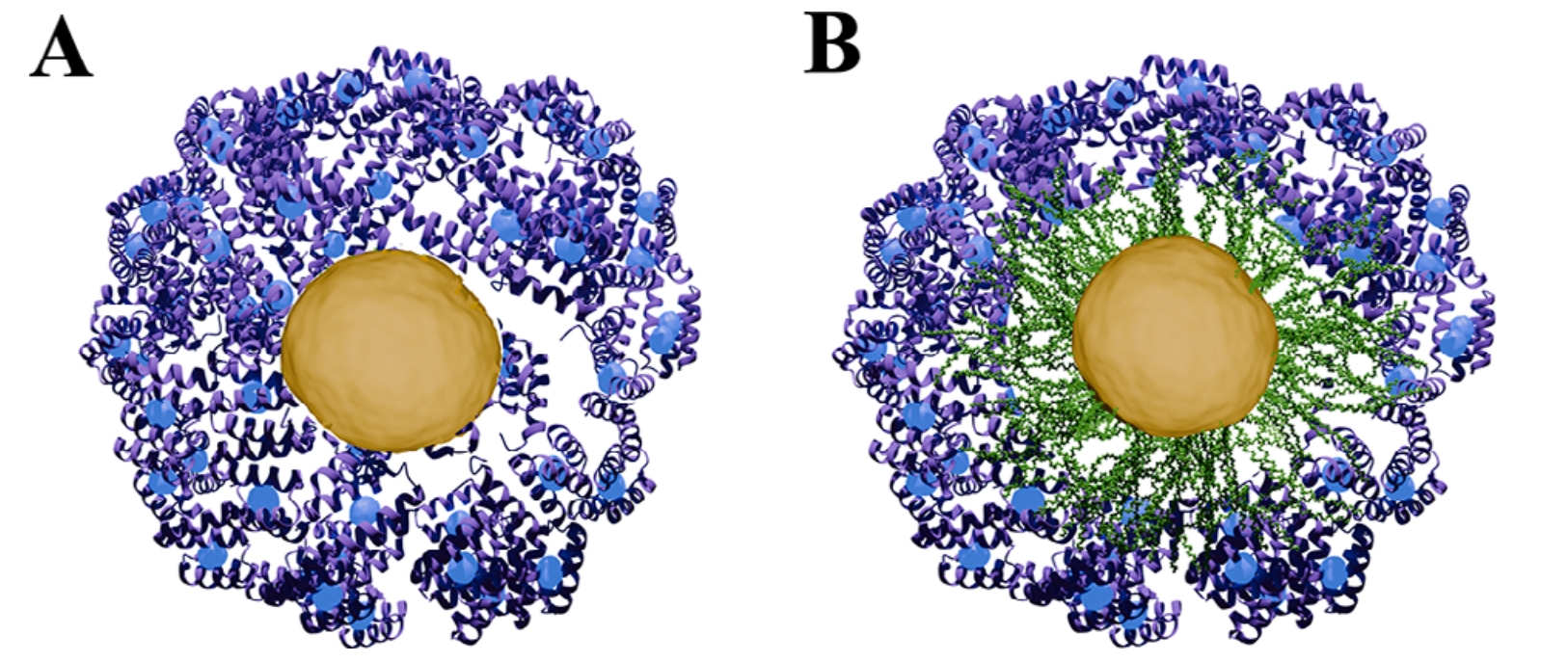
To make this work, scientists tagged different parts of gold nanoparticles with elements that strongly interact with X‑rays: the gold core was already a good candidate, but they also added bromine atoms to the polymer coating and gadolinium to the proteins forming the corona. With this clever labelling, ASAXS could separate the scattering signal of the core, the coating, and the protein layer.
The results were striking. The team confirmed, with excellent agreement to light scattering and electron microscopy, that ASAXS could detect whether a protein corona had formed and even estimate its thickness. In some cases, the method revealed subtle behaviours that other techniques missed—for instance, when proteins did not form a clear shell but instead nestled within the polymer layer.
Of course, the method has its challenges. It requires access to a synchrotron, a facility that produces powerful X‑ray beams, which is not something every laboratory can use. It also depends on adding heavy atoms such as bromine or gadolinium, which might not always be compatible with the intended medical use of a nanoparticle. Still, the promise is significant: ASAXS can analyse nanoparticles in solution, in hydrated conditions, and potentially even inside biological tissues without the need for drying, staining, or cutting thin sections.
Nanoparticles in their true working environment
The broader implication is that we are moving closer to tools that let us watch nanoparticles in their true working environment. Instead of relying only on indirect clues or on “frozen” snapshots, researchers can now probe how nanoparticles evolve in the body: how coatings protect them, how proteins attach, and how their size and shape change with time.
Why does this matter for medicine? Because the effectiveness of nanoparticle‑based therapies depends not just on what we design in the lab, but on what the body makes of them. A drug‑delivery system that works beautifully in a test tube may fail once proteins from blood reshape its surface. Conversely, a particle that looks unstable in the lab may become surprisingly stable inside tissues. With methods like ASAXS, we have a much better chance of predicting these outcomes, improving safety, and designing smarter nanomedicines.
In the coming years, as X‑ray techniques become more accessible and portable, we may well see ASAXS or related approaches used not just for research but in clinical settings, guiding how nanoparticles are tailored for individual patients. The dream is to move from trial and error toward rational design, where we can anticipate how a particle will behave inside the body and adjust it accordingly.
Nanoparticles are not just tiny tools; they are dynamic players in a complex biological game. Thanks to advanced X‑ray methods, we are finally learning the rules.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Marvin Skiba, Lars Klemeyer, Gabriela Guedes, Xiao Sun, Sylvio Haas, Aitziber L. Cortajarena, Dorota Koziej, Wolfgang J. Parak, and Carlos Sanchez-Cano (2025) Probing the Biological Identity of Inorganic Nanoparticles with Anomalous Small Angle X-Ray Scattering Small doi: 10.1002/smll.202504135 ↩