Unlocking the secrets of recyclable vitrimers
Unlocking the secrets of recyclable vitrimers
In polymer science, the classic division is between thermoplastics, which soften and flow when heated, and thermosets, which are permanently cross-linked and keep their shape no matter how hot they get—at least until they burn. In the last decade, researchers have discovered a fascinating new class of materials called vitrimers, which sit between these two categories. A vitrimer is a cross-linked network like a thermoset, but its crosslinks can swap partners through a special type of covalent bond exchange reaction. This means the material is solid and mechanically robust under everyday conditions, but if you heat it up enough, the network can slowly reorganize without losing its integrity. That unique feature allows vitrimers to be reshaped, repaired, welded, or even recycled—something traditional thermosets cannot do.
Reversible bonds are key to vitrimers
A new work 1 explores this idea using a network built from branched polyglycerol. This polymer, which has a tree-like structure rich in hydroxyl groups, is functionalized with β-ketoester groups on its branches. Those groups react with amines to form enamine bonds, and these bonds serve as crosslinks that tie the network together. The important twist is that the enamine bonds are reversible: when you heat the network, they can undergo an associative exchange reaction, breaking and reforming in a way that keeps the network connected but rearranges which chains are linked to which. This is the molecular mechanism that gives the material its vitrimeric nature.
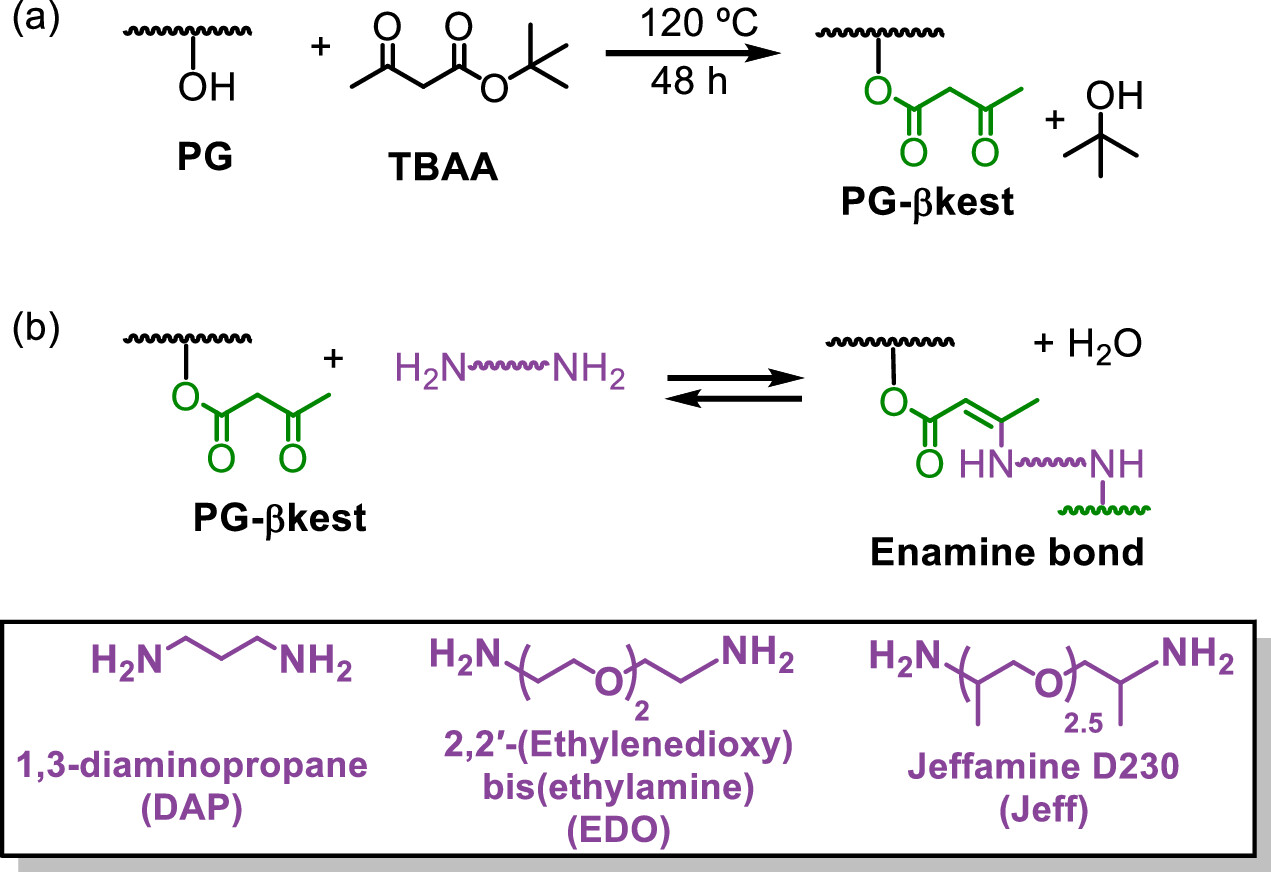
What makes this paper especially interesting is the experimental strategy the authors used to observe the network’s dynamic behavior. A common tool to study thermal transitions in polymers is differential scanning calorimetry (DSC), which heats a sample at a modest rate and records heat flow. But when bonds themselves can exchange, slow heating allows those chemical rearrangements to occur during the scan, and the thermal signal is a mixture of purely physical transitions—like the glass transition, where chain segments begin to move—and chemical relaxation caused by crosslink exchange.
Fast scanning calorimetry
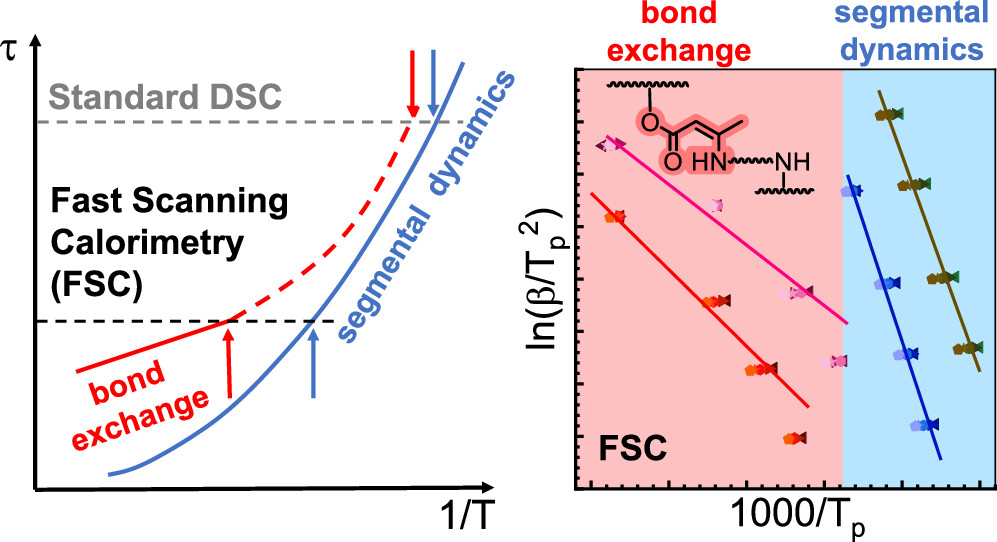
To separate these effects, the authors turned to fast scanning calorimetry (FSC), a chip-based calorimetric technique that can heat and cool extremely quickly. By heating orders of magnitude faster than conventional DSC, FSC can “outrun” the slow chemical processes and show what the network looks like if the bonds do not have time to exchange during the scan. This comparison between fast and slow scans lets the researchers distinguish the intrinsic glass transition of the network from the additional relaxation introduced by dynamic bond exchange, and it allows them to extract information about the kinetics of those exchanges. Over the last decade, FSC has become a powerful way to study crystallization, glass transitions, and other rapid thermal phenomena in polymers, and here it proves especially valuable for studying a dynamic covalent network.
Speed is key
The results show that the network’s thermal behaviour depends strongly on how quickly it is heated. At slow rates, the enamine bonds can rearrange as the sample passes through its glass transition, and the heat flow signal reflects both the physical transition and the gradual reorganization of the network. At very fast rates, the rearrangement cannot keep up, and what appears is a “frozen” picture of the network’s intrinsic glass transition. This difference is crucial because it allows the authors to quantify how fast the exchange reactions are and how much energy they require to proceed. By fitting their data, they extract kinetic parameters such as activation energies, which tell us in what temperature range the network begins to relax and how rapidly it does so once activated.
The picture that emerges is of a material that is stable at room temperature but can be activated to relax and flow when heated to a higher, controlled temperature. The authors also show that changing the crosslink density or the precise amine used to make the enamine bonds can shift this behaviour, demonstrating that both chemistry and architecture control the balance between rigidity and reprocessability.
A practical design principle
This is more than just an elegant experiment: it highlights a practical design principle. To be useful, a vitrimer must resist unwanted creep under normal conditions but still be able to flow when you intentionally heat it for reshaping or recycling. Measuring the network’s response at very fast and very slow heating rates provides a clearer map of this “safe operating window.” It also gives a rare view of how chemical bond exchange interacts with the polymer’s glassy dynamics, something that is usually hidden in conventional thermal or mechanical measurements.
This research is a reminder that good materials design relies on good measurement. By combining clever network chemistry with a time-sensitive probing technique, they shed light on how a vitrimer’s molecular bonds govern its macroscopic properties. This deeper understanding will help chemists fine-tune vitrimers for applications where strong, lightweight, and reprocessable materials are needed—from coatings to adhesives to structural components—helping to close the loop toward more sustainable plastics.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Vasiliki Maria Stavropoulou, Marta Aldecoa-Ortueta, Ester Verde-Sesto, Valerio Di Lisio, Anabel Lam, José A. Pomposo, Angel Alegría, Daniele Cangialosi, and Fabienne Barroso-Bujans (2025) Vitrimeric Behavior Revealed by Fast Scanning Calorimetry in Branched Polyglycerol Networks Cross-Linked by Reversible Enamine Bonds Macromolecules doi: 10.1021/acs.macromol.5c01560 ↩