How quantum heat engines gain efficiency by staying out of balance
How quantum heat engines gain efficiency by staying out of balance
One of the most fascinating ideas in physics is how we can turn heat into useful work. This is the basic principle behind all sorts of engines, like the ones that power cars or generate electricity in power plants. For over a hundred years, scientists have relied on the laws of thermodynamics to figure out just how efficient these engines can be. These rules set strict limits based on the temperatures involved, and they’ve held up remarkably well in the everyday world. But in recent years, researchers have started exploring what happens when you build engines on a tiny, quantum scale, using things like atoms or electrons instead of pistons and steam. A new study 1 takes this even further, revealing that in some quantum setups, engines can actually work better when they don’t fully settle into the balanced state we call thermal equilibrium.
Heat engines
Imagine you have two places at different temperatures: one hot and one cold. The engine pulls heat from the hot side, uses some of that energy to do work (like spinning a wheel or generating power), and dumps the leftover heat into the cold side. In the classical world, there’s a famous limit to how much work you can squeeze out, set by the temperature difference. It doesn’t matter how you design the engine; you can’t beat that bound.
Now, shift to the quantum realm. Here, the “working substance” isn’t a gas expanding in a cylinder but a quantum system with discrete energy levels, following the weird rules of quantum mechanics. A popular model is the quantum Otto engine, which mimics the cycles in a car engine but on a microscopic level. It goes through steps where the system’s energy levels are adjusted without adding or removing heat, and other steps where it connects to a heat source or sink to exchange energy without doing mechanical work.
Normally, we’d assume that when the quantum system touches a heat bath (a kind of thermal reservoir), it eventually reaches full thermal equilibrium. In that state, everything evens out to a single temperature, and the system forgets its starting point, except for basics like total energy. But quantum systems often have extra “conserved quantities”, properties that don’t change over time, like certain symmetries or charges. These extras can block the path to full equilibrium, even after a long wait.
The prethermal state
What happens instead is something called a prethermal state. It’s like a halfway house: the system stabilizes quickly in some ways, looking calm and steady, but it’s not truly at equilibrium because those conserved quantities keep restricting how energy moves around. These prethermal states can last a really long time compared to the system’s usual rhythms, making them practical for running an engine.
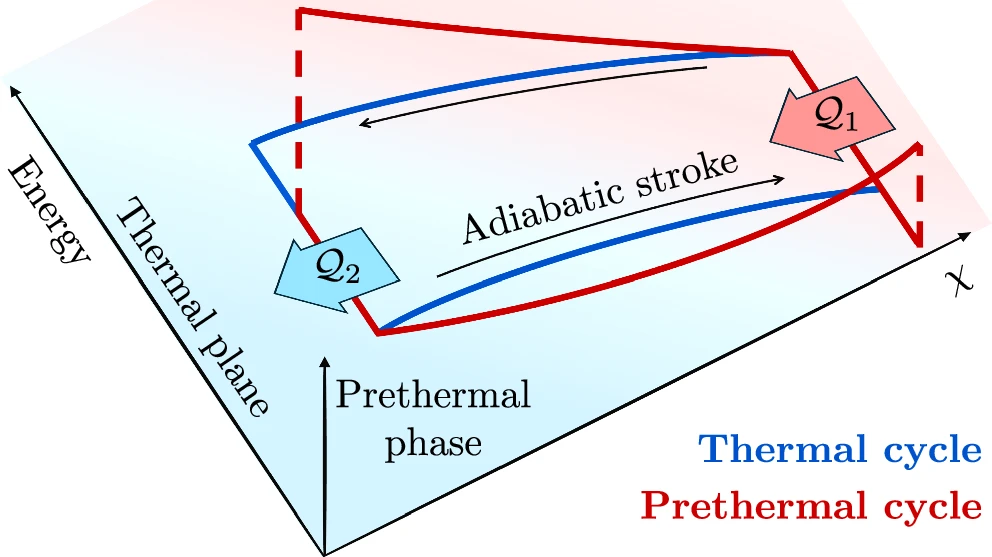
The researchers in this study looked at quantum heat engines where the working medium has these extra conservation laws, operating in prethermal states rather than full equilibrium. They focused on the Otto cycle and found something intuitive at first: when the engine runs between two normal heat baths at positive temperatures, those extra constraints hurt efficiency. The system can’t fully adjust to the baths, so you lose some potential work. That makes sense, limitations usually mean less performance.
Negative absolute temperatures
But the twist comes in a stranger regime: negative absolute temperatures. This isn’t as crazy as it sounds. In physics, negative temperature happens in systems with a cap on their energy levels, like spins in a magnetic field. It’s not colder than zero; it’s actually hotter than any positive temperature state. Picture it as having more particles in high-energy spots than low ones, an inverted population full of extra energy ready to burst out.
In this negative-temperature setup, the study shows the opposite effect. Prethermalization actually boosts the engine’s efficiency beyond what you’d get with full thermalization. This isn’t a fluke or something that needs perfect tweaking; it’s a general result from thermodynamic principles applied to these constrained quantum systems. The extra conserved quantities help the engine extract more work from the heat it absorbs.
To back this up, the team used both broad theoretical arguments and specific calculations in quantum models that are well-understood, like the Ising model and the XXZ chain. These are integrable systems, meaning they can be solved exactly, and they used tools like Generalized Hydrodynamics to track how energy and conserved quantities flow during the cycle. Even for real, finite engine cycles (not just tiny, idealized ones), the efficiency gain holds strong in the negative-temperature regime.
Staying out of balance
This discovery flips a long-held view in thermodynamics: equilibrium isn’t always the gold standard for performance. In the quantum world, staying away from full balance can sometimes be an advantage. It’s especially intriguing because negative temperatures aren’t just theory; they’ve been created in labs with things like ultracold atoms or nuclear spins.
Building a real quantum engine like this may sound futuristic, but some experimental advances have already been made, and these ideas could shape emerging quantum technologies. As we get better at controlling quantum systems, think quantum computers or simulators, we might design tiny machines that tap into prethermal states and negative temperatures to push closer to theoretical efficiency limits. More than that, this work deepens our grasp of how thermodynamics plays out when quantum effects and out-of-equilibrium behaviors take center stage.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Brollo, A., del Campo, A. & Bastianello, A. (2025) Universal efficiency boost in prethermal quantum heat engines at negative temperature. Nat Commun doi: 10.1038/s41467-025-66424-1 ↩