Synergistic effects of electrostatic bonds in a self-assembled molecule
Synergistic effects of electrostatic bonds in a self-assembled molecule
One feature of supramolecular chemistry is that of self-assembly, in which the structure forms spontaneously as a consequence of the nature of the molecules. Weak non-covalent bonds are fundamental for designing self-assembled organic structures with potentially high responsiveness to mechanical, light, and thermal stimuli. The contributions of multiple weak interactions control the ability of the structure to adapt to external stimuli and increase the stability of the organic networks. Under their action, the cohesive energy of the system becomes greater, synergistic, than the sum of the individual contributions.
A synergistic interaction is generally expected in organic crystals with multiple secondary bonds that involve hydrogen or other atoms such as halogens, chalcogens, and pnicogens. The anisotropic distribution of charge that characterizes these atoms promotes highly-directional electrostatic bonds controlled by long-range interaction between regions with positive and negative potentials. Consequently, the interatomic separations of secondary bonds are larger than the length of a covalent bond, but shorter than the sum of van der Waals atomic radii.
The directionality of the bonds and the multiplicity of the interactions involved result in characteristic arrangements of interacting molecules, called synthons. These supramolecular units shape the crystal structure, define the charge transport and the catalytic properties and are fundamental in drug design and in biochemistry.
Interestingly, weak interactions allow for crystal design and engineering of its specific electronic, mechanical and lattice dynamic properties under mild conditions. These secondary bonding interactions in two-dimensional molecular assembly are of interest for fundamental studies and for applied optoelectronics, from photovoltaics, photoluminescence or sensing to organic-based electronics, or medical diagnostics. Still, very few works have addressed their synergistic role. Indeed, very often, these interactions are masked by strong covalent bonds in metal–organic assemblies, or by the interaction with the supporting metal surfaces.
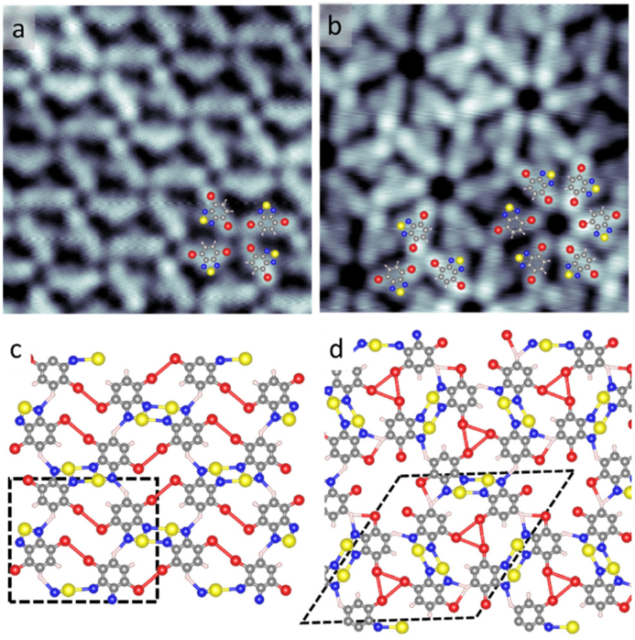
Now, a team of researchers reports 1 on the multiple secondary bonds leading to the assembly of one molecular species in two ordered networks supported on a Au(111) surface. The team shows that, favoured by the weak molecular interaction with the Au surface, the cohesion of the two assemblies is driven by the synergistic interaction of sulphur, nitrogen, hydrogen and bromine atoms.
In the molecular species, 4,7-dibromo-benzo[c]-1,2,5-thiadiazole (2Br-BTD), at first the interactions of sulphur and nitrogen atoms lead to the pairing of molecules through the thiadiazole group, which is clearly a common trend in both networks. However, the weak multiple secondary bonds promote also the formation of characteristic supramolecular units that form synthons.
The researchers use low-temperature scanning tunnelling microscopy and density functional theory calculations to demonstrate that multiple secondary-interactions strengthen the electrostatic attraction between the pnicogen and chalcogen atoms forming [S–N]2 heterocycles, the building block of the two networks. Under the synergistic contribution of additional dipolar interactions, such as those between halogen–halogen, nitrogen–hydrogen or hydrogen–bromine atoms, the [S–N]2 interatomic distance decreases in the two molecular networks, increasing the cohesion of the system.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Ana Barragán, Sara Lois, Ane Sarasola and Lucia Vitali (2022) Empowering non-covalent hydrogen, halogen, and [S–N]2 bonds in synergistic molecular assemblies on Au(111) Nanoscale doi: 10.1039/D2NR05984C ↩