The stabilizing effect of a binder for transition metal oxides to sustain water oxidation in acidic environments
The stabilizing effect of a binder for transition metal oxides to sustain water oxidation in acidic environments
Global energy demand is expected to rise around 30% by 2040 according to the International Energy Agency (IEA). Hydrogen (H2) produced by the electrolysis of water, using renewable electricity, the so-called green hydrogen, has emerged as a promising energy vector to respond to this increasing energy demand with the potential to decarbonize transportation, heating, and fine chemicals sectors. However, current technologies used to split water use expensive catalysts in extreme pH conditions that involve environmental pollution risks and handling hazards.
Efficiency is related to the concept of overpotential, the potential that must be applied in an electrolytic cell in addition to the theoretical potential required to liberate a given substance at an electrode. Hence, the smaller the overpotential, the better. One of the challenges in water electrolysis remains the oxidation catalysts for the anodic oxygen evolution reaction (OER). This 4-electron process is one of the main causes for the slow kinetics and high overpotentials.
Numerous earth-abundant materials are able to work as OER electrocatalysts in alkaline media, reaching excellent performance, for instance the family of nickel–iron mixed oxides or (oxo) hydroxides. But alkaline technologies have several disadvantages, such as the need for corrosive media (hot, concentrated KOH solutions), and the relatively low current densities achieved, as limited by the OH− transport through the separator/anion-exchange membrane.
Significantly higher current densities can be achieved under acidic conditions, thanks to the ultrafast proton transport through a proton exchange solid electrolyte (membrane) and the high proton concentration available for hydrogen generation (HER). However, only IrO2-based catalysts are efficient and stable under these conditions. At low pH, inexpensive metal oxides suffer serious dissolution and deactivation, particularly when working at high potentials/current densities.
Recently, a team of researchers demonstrated 1 that the use of a hydrophobic binder in the anode composition is able to stabilize Co3O4, otherwise unstable under these conditions. A Co3O4/CPO (graphitic carbon + paraffin oil) composite sustained a current density of up to 100 mA cm−2 in a 1 molar H2SO4 electrolyte. This very same electrode architecture cannot be scaled up to industrial applications, given the intrinsic long-term instability of carbon conducting supports under the OER. But the results confirm the validity of this approach to stabilize the active catalysts at low current densities.
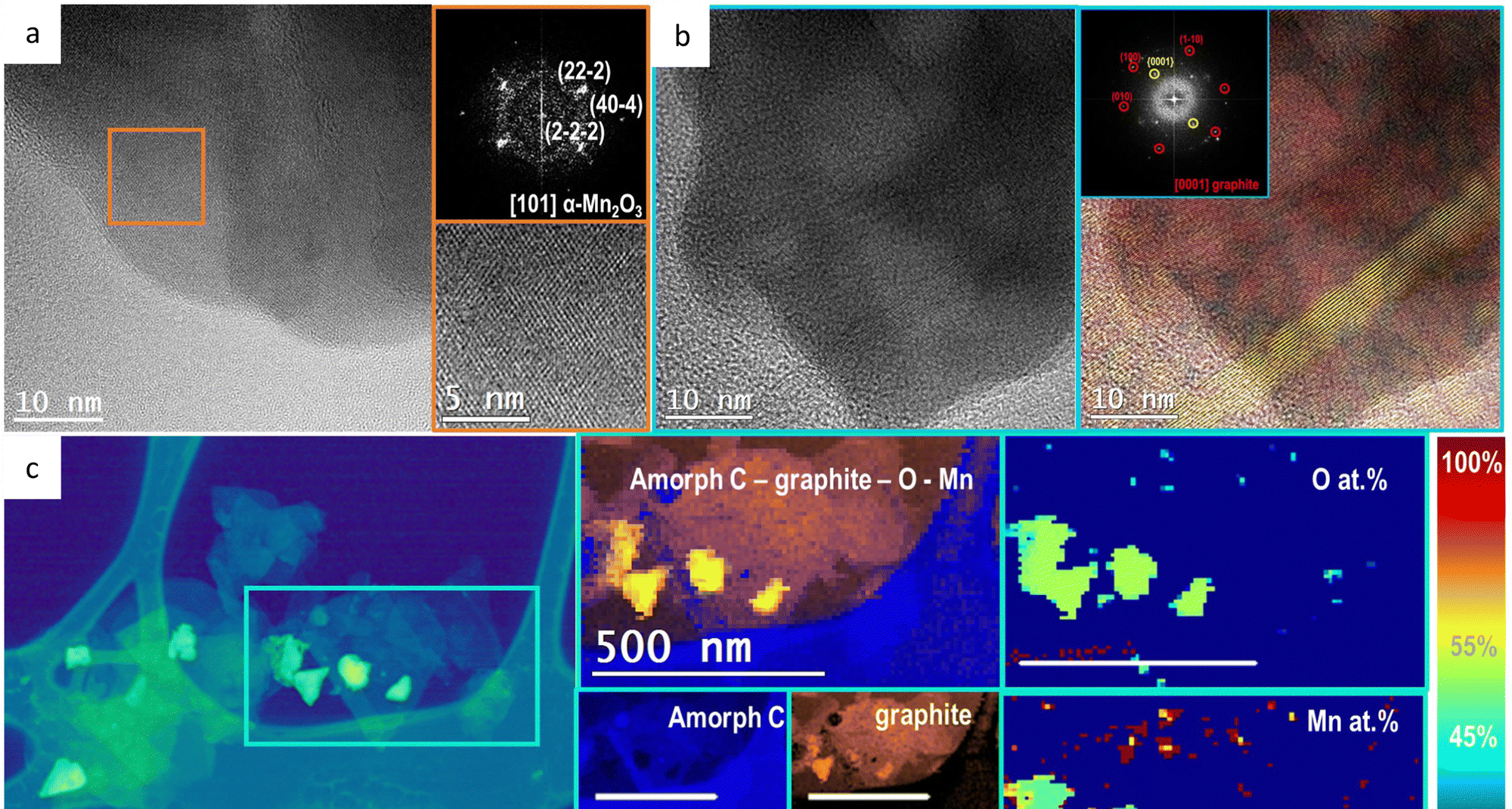
Taking advantage of this, the same team now reports 2 a wide screening of first row transition metal (Mn, Fe, Co, Ni and Zn) oxides with mono-, bi- and trimetallic equimolecular composition as OER catalysts in 1 molar H2SO4.
Interestingly, they find that even Mn-based oxides appear stabilized, while otherwise were characterized as highly unstable. Protected by the paraffin oil, Mn2O3 anodes showed the best performance in terms of lower overpotentials to reach 10 mA cm−2, with excellent stability (>24 h) when working in 1 M sulfuric acid solution without a sign of fatigue or deactivation.
The incorporation of a second or third metal did not improve the activity of the Mn-based oxide. Conversely, the Mn doping was critical to improve the OER activity of some other oxides, such as FeOx, NiOx and FeNiOx, with >120 mV overpotential decrease at 10 mA cm−2 current density.
These results confirm the general stabilizing effect of a partially hydrophobic, conducting binder to allow earth-abundant transition metal oxides to sustain water oxidation in acidic environments.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- J. Yu , F. A. Garcés-Pineda , J. González-Cobos , M. Peña-Díaz , C. Rogero , S. Giménez , M. C. Spadaro , J. Arbiol , S. Barja and J. R. Galán-Mascarós (2022) Sustainable oxygen evolution electrocatalysis in aqueous 1 M H2SO4 with earth abundant nanostructured Co3O4Nat. Commun. doi: 10.1038/s41467-022-32024-6 ↩
- Jiahao Yu, Stefano Giancola, Bahareh Khezri, David Nieto-Castro, Jesús Redondo, Frederik Schiller, Sara Barja, Maria Chiara Spadaro, Jordi Arbiol, Felipe A. Garcés-Pineda and José Ramón Galán-Mascarós (2023) A survey of Earth-abundant metal oxides as oxygen evolution electrocatalysts in acidic media (pH < 1) EES Catalysis doi: 10.1039/d3ey00101f ↩