Extracting lithium from waste liquids using aluminium hydroxide
Extracting lithium from waste liquids using aluminium hydroxide
A team of researchers has invented 1 a more efficient way to extract lithium from waste liquids leached from mining sites, oil fields and used batteries. They demonstrated that a common mineral can adsorb at least five times more lithium than can be collected using previously developed adsorbent materials.
The new low-cost high-lithium-uptake process presents the key advantage of working in a wider pH range of 5 to 11 compared to other direct lithium extraction methods. The acid-free extraction process takes place at 140 degrees Celsius, compared to traditional methods that roast mined minerals at 250 degrees Celsius with acid or 800 to 1000 degrees Celsius without acid.
Lithium is a lightweight metal commonly used in energy-dense and rechargeable batteries. Electric vehicles, which are needed to achieve net-zero emissions by 2050, rely on lithium-ion batteries. Industrially, lithium is extracted from brines, rocks and clays. The new process may help meet rising demand for lithium by making domestic sources commercially viable.
The research reveals a route away from the statu quo: a linear economy in which materials from mining, refining or recycling are made into products that, at the end of their lives, are discarded as waste. The work moves toward a circular economy in which materials are kept in circulation as long as possible to reduce consumption of virgin resources and generation of waste.
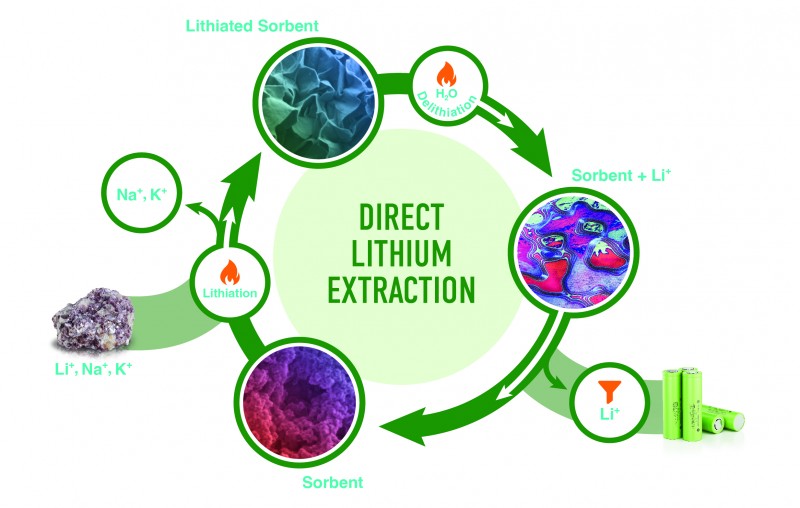
The new process relies on aluminium hydroxide, a mineral that is abundant in Earth’s crust. The scientists used aluminium hydroxide as a sorbent, which is a material that takes up another material — in this case, lithium sulfate — and holds it.
In a process called lithiation, an aluminium hydroxide powder extracts lithium ions from a solvent to form a stable layered double hydroxide, or LDH, phase. Then in delithiation, treatment with hot water causes the LDH to relinquish lithium ions and regenerate the sorbent. During relithiation, the sorbent is reused to extract more lithium. 2
Aluminium hydroxide exists in four highly ordered crystalline polymorphs and one amorphous, or disordered, form. Form turns out to play a big role in the sorbent’s function. Because amorphous aluminium hydroxide is the least resulted to be the less stable among the mineral’s forms, it spontaneously reacts with lithium from brine leached from waste clays.
The researchers used scanning electron microscopy to characterize the morphology of aluminium hydroxide during lithiation. It is a charged neutral layer that contains atomic vacancies, or tiny holes. Lithium is absorbed at these sites. The size of these vacancies is the key to aluminium hydroxide’s selectivity for lithium, which is a positively charged ion, or cation.
The selectivity of amorphous aluminium hydroxide for lithium results in near-perfect efficiency. In a single step, the process captured 37 milligrams of lithium per gram of recoverable sorbent — approximately five times more than a crystalline form of aluminum hydroxide called gibbsite, which was previously employed for lithium extraction. The first step of lithiation extracts 86% of the lithium in the leachate, or brine, from mining sites or oil fields. Running the leachate through the amorphous aluminium hydroxide sorbent a second time picks up the rest of the lithium. In two steps, you can fully recover the lithium.
Calculations showed that the new technology used one-third the material and one-third the energy and subsequently generated fewer greenhouse gas emissions than the current procedure.
Next, the researchers want to extend the process to extract more lithium and regenerate the sorbent in a specific form. Now, when the amorphous aluminium hydroxide sorbent reacts with the lithium and is later treated with hot water to remove the lithium and regenerate the sorbent, the result is a structural change in the aluminium hydroxide polymorph from amorphous to a crystalline form called bayerite.
Success in optimizing the new process for extraction speed and efficiency could be a game-changer for the domestic lithium supply. More than half of the world’s land-based lithium reserves are in places where the concentration of dissolved minerals is high.
References
- K. Jayanthi et al (2023) Integrated Circular Economy Model System for Direct Lithium Extraction: From Minerals to Batteries Utilizing Aluminum Hydroxide ACS Appl. Mater. Interfaces doi:10.1021/acsami.3c12070 ↩
- K. Jayanthi et al (2023) Effect of Anions on the Delithiation of [Li–Al] Layered Double Hydroxides: Thermodynamic Insights The Journal of Physical Chemistry doi:10.1021/acs.jpcc.3c05676 ↩