The sweet spot of self-sufficient heterogeneous biocatalysts
The sweet spot of self-sufficient heterogeneous biocatalysts
Authors: Fernando López-Gallego, Principal Investigator, Ikerbasque Professor and group leader at the Heterogeneous Biocatalysis Laboratory at CIC biomaGUNE; Ainhoa Oliden-Sánchez, Postdoctoral Researcher at the Heterogeneous Biocatalysis Laboratory at CIC biomaGUNE; Clara García-Gorro, Science Communication Manager at CIC biomaGUNE.
Self-sufficient heterogeneous biocatalysts are emerging as powerful tools for greener, more efficient chemical manufacturing. By cleverly combining enzymes and their essential cofactors in one material, they promise to simplify processes and reduce costs. But what makes them truly effective?
Researchers at CIC biomaGUNE working in collaboration with the Donostia International Physics Center (DIPC) have discovered that a classic principle from heterogeneous catalysis also applies to cofactor binding in immobilized biocatalysts.
A new generation of enzymes for green manufacturing
Enzymes are changing the way we make chemicals. As they are highly selective and work best at mild temperatures, near neutral pH, atmospheric pressure, and prefer water-based environments rather than harsh solvents, enzymes offer a more environmentally friendly alternative to traditional catalysts used in synthetic chemistry and industrial processes. This approach, known as applied biocatalysis, is already being used in chemical manufacturing, but mostly with enzymes that don’t need exogenous cofactors to function. These exogenous cofactors are mostly organic molecules that act as co-catalysts, being essential for enzyme catalysis. They interact with the enzyme’s active site reversibly, entering and exiting the active site in each reaction cycle. Inside the cells, these cofactors are continuously synthesized and recycled, as well as compartmentalized together with the enzymes that use them.
In recent decades, researchers have uncovered numerous cell-free enzymatic reactions that do require the presence of these exogenous cofactors. However, their implementation in vitro is extremely challenging because of their high cost and inherent instability when they are out of the cells, limiting their industrial exploitation.
One promising strategy to address this issue involves stabilizing these cofactors and co-immobilizing them together with their enzyme partner on the same support material. This approach allows the cofactors to be reused repeatedly, without being exogenously added to the reaction, thereby enhancing the overall efficiency and sustainability of the entire catalytic system.
Building on this concept, our group, along with others in the field, has recently developed a novel class of biocatalysts known as self-sufficient heterogeneous biocatalysts (ssHBs) 1.
The term “self-sufficient” refers to the fact that these enzymes do not rely on an external supply of cofactors, which can significantly reduce costs in chemical manufacturing. Meanwhile, “heterogeneous” describes the presence of two different phases: the biocatalyst is immobilized on a solid support, whereas the substrate and product are in the liquid phase.
How do self-sufficient heterogeneous biocatalysts work?
These innovative systems consist of enzymes co-immobilized with their specific cofactors on porous materials. More specifically, the enzyme is irreversibly immobilized on porous materials, while the cofactor is immobilized reversibly. In this way, the cofactor can diffuse from the carrier surface to the anchored enzymes through the intraporal space, while its interaction with the surface hampers its leaching to the reaction media.
To better understand how self-sufficient heterogeneous biocatalysts work, we illustrate this concept through the following analogy. Imagine a person (the enzyme) is anchored to the ground of a water tank by a harness so that they cannot break free and float in the water. This person wants to open a floating jar (the substrate to transform) when the tank is full of water. To do so, the person must take a jar opener (the cofactor) to open the jar (the reaction). If the tank is full of water, the jar opener floats and it is inaccessible for the person to catch it unless this is bound to the bottom of the water tank through a magnet, allowing it to be semi-fixed rather than completely stationary. In this scenario, the jar opener is sometimes anchored to the floor by the magnet, but at other times it floats freely around the room. This dynamic enables the enzyme to access the cofactor as needed to carry out its function.
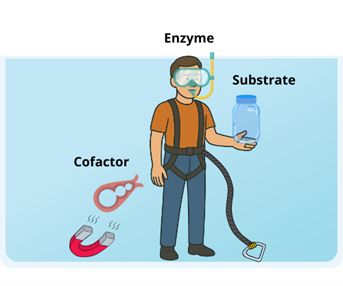
Although these self-sufficient heterogeneous biocatalysts (ssHBs) have been used in the lab to improve how cofactors are reused, we still don’t fully understand how they work.
Finding balance: the Sabatier principle in self-sufficient heterogeneous biocatalysis
We hypothesized that there is a dynamic balance inside the porous support between bound and unbound cofactor populations that allows the cofactor to be accessible to the enzyme while preventing it from leaking away into the surrounding solution.
We were inspired by the work of Paul Sabatier, a renowned French chemist who received the Nobel Prize in Chemistry in 1912 for his contributions to the field of catalysis. Among his contributions, Sabatier is best known for formulating a principle that now bears his name: the Sabatier principle.
This principle states that, for a catalyst to be effective, it must bind to its reactants (in this case, the substrate) with just the right strength: not too tightly, and not too weakly. If the binding is too strong, the catalyst may hold onto the reactants and block the reaction from proceeding. If it’s too weak, the reactants may not interact sufficiently to trigger the reaction.
Inspired by this idea, we wondered whether this could apply to the way cofactors bind to self-sufficient heterogenous biocatalysts.
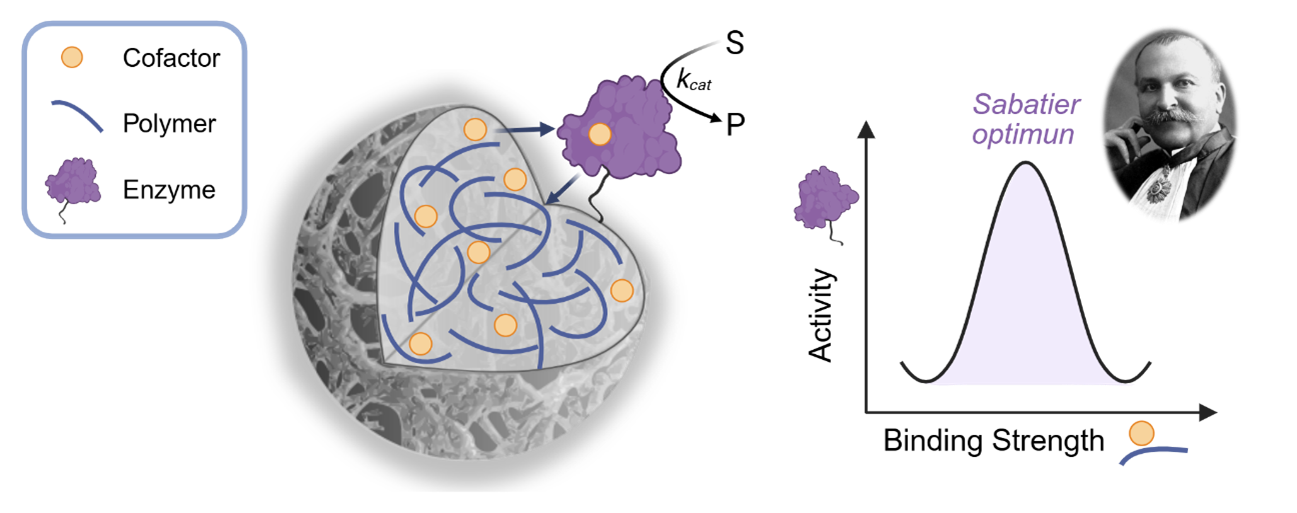
Building on the analogy introduced earlier, when the tank is filled with water, the jar opener (representing the cofactor) is sometimes anchored to the floor by a magnet, and at other times it drifts freely through the water. The capacity of the person to use the jar opener depends on the strength of the magnet, which represents the binding energy of the cofactor to be bound to the surface of the heterogeneous biocatalyst.
This dynamic equilibrium between bound and unbound states allows the enzyme to access and use the cofactor, enabling reaction turnovers. If the opener were permanently fixed to the floor, the enzyme wouldn’t be able to reach it. On the other hand, if there were no magnetic attractions at all, the cofactor would be swept away by the water and lost, making it impossible for the enzyme to function. Hence, only when the cofactor is bound to the surface of the carrier with an intermediate binding energy, the immobilized enzyme exhibits its maximum activity. Then we can say that the ssHB is working at its Sabatier optimum.
From theory to the lab
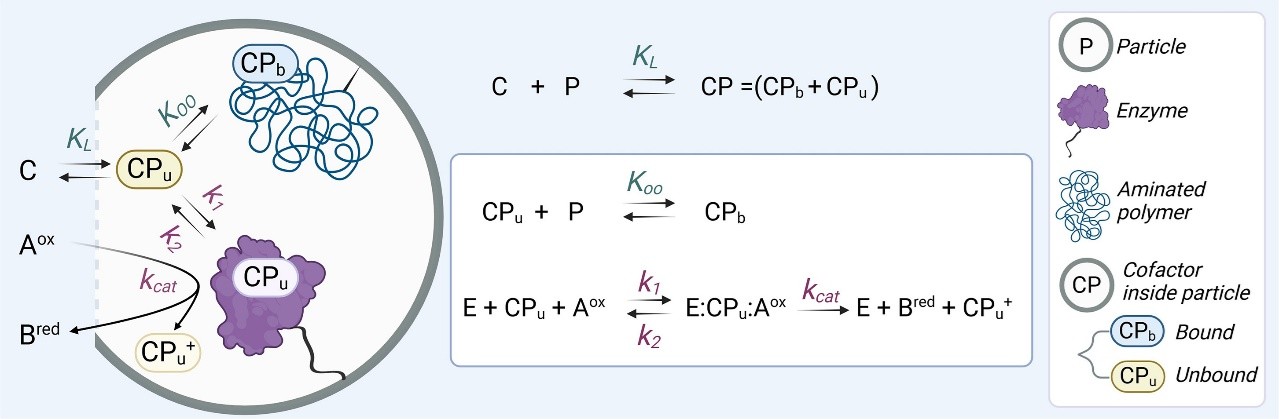
To quantify the phenomenon occurring inside the ssHB, we developed a theoretical model describing the interactions between irreversibly immobilized alcohol dehydrogenases and their cofactor NAD(P)H within a polymer confined in porous materials.
The model includes two key equilibria: one that governs how strongly the cofactor binds to the polymer, and another that determines its tendency to diffuse out of the material. These two factors directly influence how much cofactor is accessible to the enzyme and, consequently, the apparent catalytic activity of the biocatalyst.
To validate the model experimentally, we fabricated different ssHBs with common architectures. Then, we tested them under various reaction conditions where cofactor binding thermodynamics might be altered.
As model systems, we selected two enzymes that depend on the cofactor NAD(P)H: BsADH (an alcohol dehydrogenase from the bacterium Bacillus stearothermophilus) and LkKRED (a ketoreductase from the bacterium Lactobacillus kefir). We immobilized the enzymes on agarose-based beads functionalized with cobalt chelates and epoxy groups (AG-Co2+/E) and coated them with polyallylamine (PAH) and poly(ethylenimine) (PEI) to form a cationic polymer layer where this phosphorylated cofactor negatively charged can bind reversibly through electrostatic interactions.
Our results demonstrated that ssHBs obey the Sabatier principle. This highlights the crucial role of cofactor binding thermodynamics in optimizing biocatalysis for chemical applications. By tuning the cofactor’s binding strength to an intermediate level, we can maximize enzyme activity.
Thus, these findings not only help explain how ssHBs function but also give us useful design strategies to adapt these biocatalysts to different chemical processes and industrial setups.
The BRTA is a consortium that remains a step ahead of future socio-economic challenges worldwide and in the Basque Autonomous Community; it addresses them through research and technological development, thus projecting itself internationally. The BRTA centres collaborate to generate knowledge and transfer it to Basque society and industry so as to make them more innovative and competitive. The BRTA is an alliance of 17 R&D centres and cooperative research centres with the support of the Basque Government, the SPRI and the Chartered Provincial Councils of Araba, Bizkaia and Gipuzkoa.
References
- Eleftheria Diamanti, Ainhoa Oliden-Sánchez, Daniel Grajales-Hernández, Daniel Andrés-Sanz, Rut Fernández-Marín, Daniel Padro, Jesús Ruíz-Cabello, Ronen Zangi, Fernando López-Gallego (2025) The Sabatier principle governs the performance of self-sufficient heterogeneous biocatalysts for redox biotransformations Cell Reports Physical Science doi: 10.1016/j.xcrp.2025.102694 ↩
