Selective reduction and its reversibility: a crystal that breathes oxygen
Selective reduction and its reversibility: a crystal that breathes oxygen
A team of scientists has discovered a new type of crystal that can “breathe”—releasing and absorbing oxygen repeatedly at relatively low temperatures. This unique ability could transform the way we develop clean energy technologies, including fuel cells, energy-saving windows, and smart thermal devices.
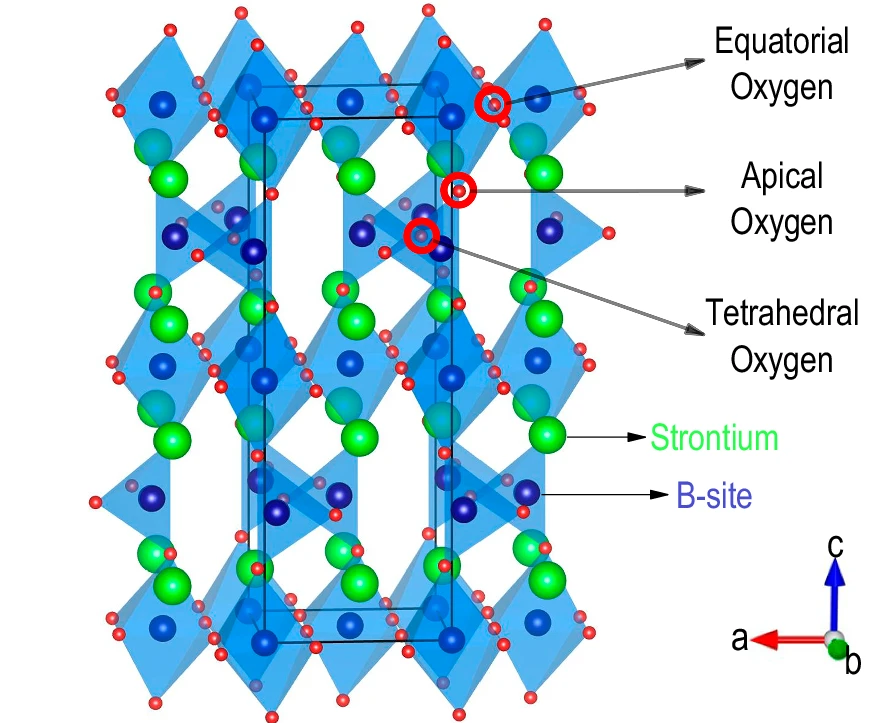
The newly developed material 1 is a special kind of metal oxide made of strontium, iron, and cobalt (formula: SrFe0.5Co0.5O2.5) . What makes it extraordinary is that it can release oxygen when heated in a simple gas environment and then take it back in, all without falling apart. This process can be repeated many times, making it ideal for real-world applications.
“It is like giving the crystal lungs and it can inhale and exhale oxygen on command,” says Prof. Jeen from the Department of Physics, Pusan National University, Korea. Controlling oxygen in materials is crucial for technologies like solid oxide fuel cells, which produce electricity from hydrogen with minimal emissions. It also plays a role in thermal transistors—devices that can direct heat like electrical switches—and in smart windows that adjust their heat flow depending on the weather.
Until now, most materials that could accomplish this kind of oxygen control were too fragile or operated only in harsh conditions like extremely high temperatures. This new material works under milder conditions and remains stable. “This finding is striking in two ways: only cobalt ions are reduced, and the process leads to the formation of an entirely new but stable crystal structure,” explains Prof. Jeen.
The researchers also showed that the material could return to its original form when oxygen was reintroduced, proving that the process is fully reversible. “This is a major step toward the realization of smart materials that can adjust themselves in real time,” says Prof. Ohta, from the Research Institute for Electronic Science, Hokkaido University, Japan . “The potential applications range from clean energy to electronics and even eco-friendly building materials.”
References
- Lee, J., Seo, YS., Pitike, K.C. et al. (2025) Selective reduction in epitaxial SrFe0.5Co0.5O2.5 and its reversibility Nat Commun doi: 10.1038/s41467-025-62612-1 ↩