Aurkines could spark a new era in fighting bile duct and resistant cancers
Aurkines could spark a new era in fighting bile duct and resistant cancers
Cholangiocarcinoma (CCA), a rare but aggressive cancer of the bile ducts, poses a major challenge for doctors and patients. It’s tough to treat, with limited options beyond surgery, and systemic drugs like chemotherapy often fall short. Cisplatin, a classic platinum-based chemo drug, works against many solid tumours but has only modest effects in CCA. Worse, cancer cells frequently develop resistance, making it even less effective over time. A recent study 1 introduces a promising new family of platinum compounds called “Aurkines” – specifically Aurkine 16 and Aurkine 18 – that work differently from cisplatin. These novel drugs show strong activity against both untreated (treatment-naïve) and cisplatin-resistant CCA cells and tumours, offering hope for better outcomes.
How cisplatin works – and why it fails
Traditional platinum drugs like cisplatin kill cancer cells by binding to their DNA and creating “crosslinks.” Think of these as sticky knots that jam up the cell’s machinery for copying and reading DNA during cell division. If the damage is too much or can’t be fixed, the cell triggers apoptosis (programmed cell death). But cancer cells are sneaky – they often ramp up DNA repair systems, pump out the drug, or detoxify it faster, leading to resistance. This is a common roadblock in treating CCA, where cisplatin is part of the standard first-line therapy but rarely leads to long-term control.
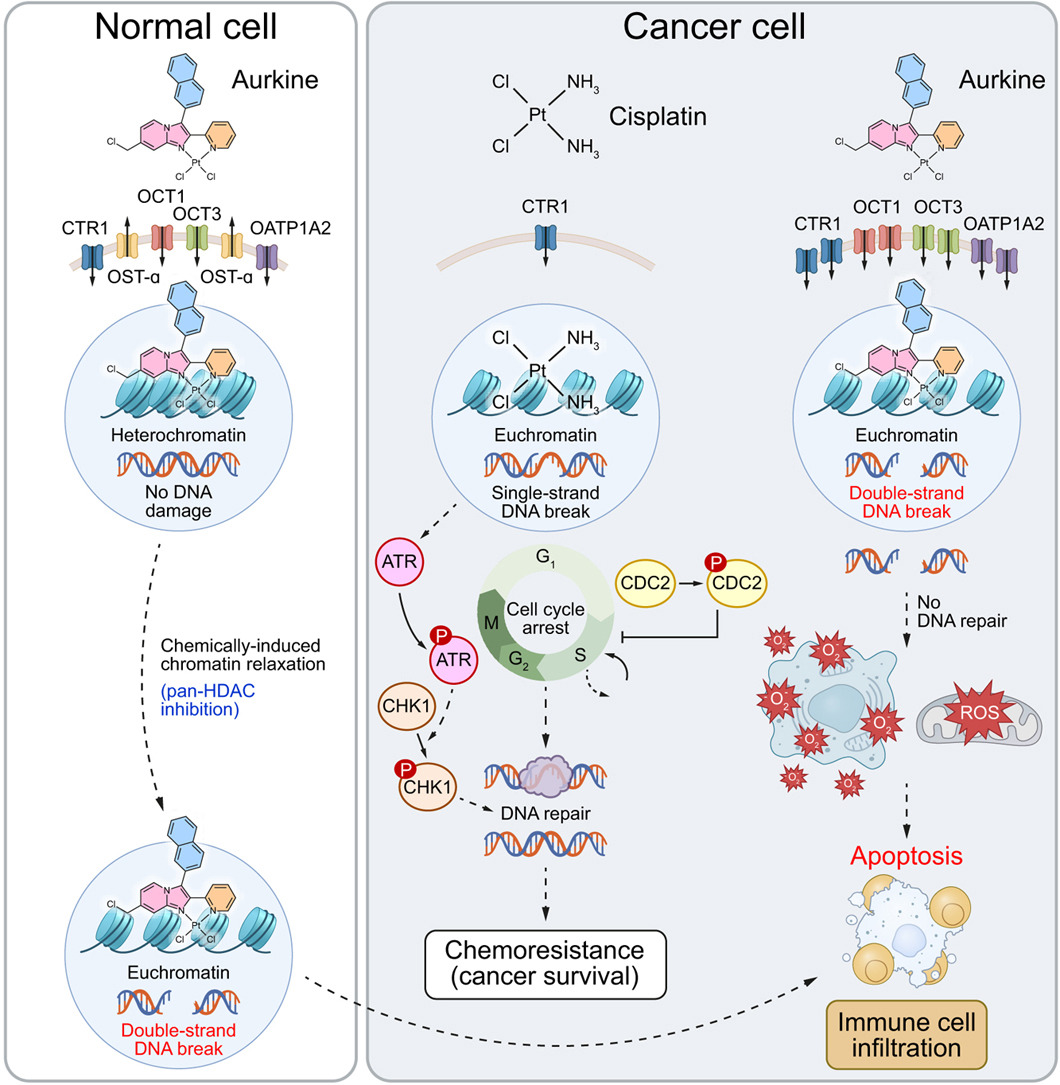
Aurkines flip the script. Instead of mostly creating those knot-like crosslinks, these compounds are designed with unique chemical properties (polyelectrophilic, meaning they interact strongly in multiple ways) to cause frequent double-strand breaks (DSBs) in DNA. DSBs are like snapping both sides of a ladder – they’re the most dangerous type of DNA damage and much harder for cells to repair. When DSBs pile up, they overwhelm the cell’s fix-it tools, pushing it straight into death pathways without giving resistance mechanisms much chance to kick in.
What the lab tests revealed
Researchers used a mix of lab techniques to prove Aurkines’ edge. Here’s a breakdown of the key findings:
- DNA damage in action: In test tubes with isolated DNA and in cultured CCA cells, Aurkines created breakage patterns visible in comet assays (a lab test where damaged DNA spreads out like a comet tail under a microscope). Microscopes and other tools confirmed widespread DSBs, along with signs of oxidative stress (harmful buildup of reactive oxygen species, or ROS) that amps up the damage.
- Killing cancer cells: The drugs reduced cell survival and triggered apoptosis in several CCA cell lines, including ones engineered to resist cisplatin. Markers like activated caspases (proteins that execute cell death) lit up, showing the process was underway.
- Sparing healthy cells: Normal bile duct cells (cholangiocytes) were far less affected at the same doses, suggesting a “therapeutic window” – enough power to hit cancer without widespread harm to healthy tissue.
- Bypassing resistance: Cisplatin-resistant cells often change how they handle drugs: less intake, more outflow, or supercharged repairs. Aurkines use different entry doors into cells, like organic cation transporters (OCT1 and OCT3), copper channels (CTR1), and OATP1A2 – not just CTR1 like cisplatin. This, plus the DSB focus, lets them sidestep common resistance tricks. While cisplatin ramps up repair signals, Aurkines drive straight to apoptosis.
The study also showed Aurkines trigger cell death in cancer-associated fibroblasts (CAFs) – supportive cells in the tumor’s neighborhood that help it grow and resist treatment. This could weaken the tumour’s defenses even more. Plus, the drugs worked against resistant cells from other cancers, like breast and ovarian, hinting at broader potential.
Proof in animal models
Building on cell studies, the team tested Aurkines in mice with CCA tumors. They used both subcutaneous (under the skin) and orthotopic (in the natural bile duct spot) models, including ones from cisplatin-resistant cells.
- Tumours shrank significantly with Aurkine treatment, showing hallmarks of DNA damage and apoptosis inside.
- In mice with working immune systems, treated tumours drew in more anti-cancer immune cells, boosting the body’s natural fight. This “immunogenicity” effect could make Aurkines even more powerful when combined with immunotherapies.
- Importantly, at effective doses, the mice showed no obvious side effects or toxicity, like weight loss or organ damage.
These results provide solid proof that Aurkines’ lab prowess translates to real tumour control in living animals.
Aurkines in perspective
Chemotherapy has come a long way, but many drugs still rely on damaging DNA to kill cancer. The trick is doing it selectively – hitting tumours hard while leaving normal cells alone. Aurkines seem to achieve this by exploiting cancer cells’ vulnerabilities: their looser chromatin (DNA packaging) lets the drugs interact more, and differences in transporters limit uptake in healthy cells.
For CCA patients, where 5-year survival rates hover below 10% for advanced cases, overcoming cisplatin resistance could be a game-changer. These compounds might not only treat fresh cases but also rescue those where standard chemo fails. The strategy could extend to other platinum-resistant cancers, addressing a huge unmet need.
Of course, no lab breakthrough is a sure thing. Mouse results don’t always predict human safety – things like drug distribution, metabolism, and rare toxicities can differ. Long-term risks from frequent DSBs, like secondary cancers, need close watching in clinical trials. The researchers stress the need for more toxicology studies and early human testing to confirm selectivity and tolerability.
In short, this study delivers compelling preclinical evidence: Aurkines cause rampant DNA double-strand breaks, spark apoptosis, and curb growth in both naïve and resistant CCA tumours in mice – all while sparing normal cells and showing no major toxicity. If these hold up in humans, Aurkines could spark a new era in fighting hard-to-treat cancers.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Irene Olaizola, Mikel Odriozola-Gimeno, Paula Olaizola, Francisco J. Caballero-Camino, Noelia Pastor-Toyos, Mireia Tena-Garitaonaindia, Ainhoa Lapitz, Beatriz Val, Amanda R. Guimaraes, Maitane Asensio, Maider Huici-Izagirre, Colin Rae, David de Sancho, Xabier Lopez, Pedro M. Rodrigues, Elisa Herraez, Oscar Briz, Laura Izquierdo-Sanchez, Aitziber Eleta-Lopez, Alexander M. Bittner (2025) New platinum derivatives selectively cause double-strand DNA breaks and death in naïve and cisplatin-resistant cholangiocarcinomas Journal of Hepatology doi: 10.1016/j.jhep.2025.04.034 ↩