Arsenic removal from water using nanoadsorbents
Water is critical for life on Earth. Our own biology, the chemistry of our bodies, is based on it. Access to clean drinking water is a human right, and is included as one of the global 17 sustainable development goals for 2030. However, today, one in three humans worldwide does not have access to safe drinking water. People in developing and developed countries alike are affected by drinking water contaminants, particularly heavy metals or pharmaceuticals. Among these contaminants, arsenic is considered one of the most dangerous hazards in drinking water, and at least 230 million people in 108 countries are drinking water containing arsenic at levels above the World Health Organization (WHO) provisional guideline value of 10 μg/L.
Many water-purification technologies have been proposed to remove arsenic. These technologies are variations of methods within the standard catalogue of chemical engineering solutions: membrane filtration, distillation, reverse osmosis, adsorption, or ion exchange. But, most require several stages, implying higher investment and operating costs, high-energy requirements, and reliance on materials with a high environmental footprint. One of them, namely adsorption by nanoparticles (nanoadsorbents), differentiates from the rest because it involves the use of materials extracted from by-products, offering high removal efficiency combined with sustainability.
One drawback of using bulk nano-adsorbents is their tendency to agglomerate, which means a drop in active surface and, hence, decreasing efficiency. Another one is their toxicity to humans and, in general, the environmental impact if they are directly used in the water system. Even biodegradable nanoparticles may accumulate within cells and lead, for example, to genetic mutations. Consequently, if nanoparticles are to be used, keeping them from output water becomes a central problem and their confinement in a polymeric matrix a solution.
For arsenic removal, iron oxide nanoparticles are excellent adsorbents, with high selectivity. A particular way to immobilize these iron oxide nanoparticles, instead of using them in bulk, is via electrospinning, a versatile and scalable membrane fabrication technique, which allows the production of nano- and submicron polymeric fibres.
A suitable polymer to prepare membranes using electrospinning is poly(vinyl alcohol) (PVA). PVA is biodegradable, which is a plus when dealing with water purification. From a technical point of view it fulfils a necessary feature for electrospinning, it can dissolve in several solvents, and particularly in water. But, more importantly, PVA can crosslink or dehydrate to become insoluble without losing its swelling capacity. The membranes must swell during the remediation process, allowing contaminants to reach the nanoparticles. The success of the procedure depends entirely on the swelling of the membranes.
Generally, preparing nanoadsorbent membranes involves two steps: the synthesis of the nanoparticles themselves and their dispersion in the polymeric matrix. However, this procedure raises other issues, such as the significant size of the nanoparticles, the limitation of their quantity included in the polymer, and their dispersion within the polymer. Why not making everything in one step? That is, would in-situ synthesis of iron oxide nanoparticles during the electrospinning process into PVA be feasible?
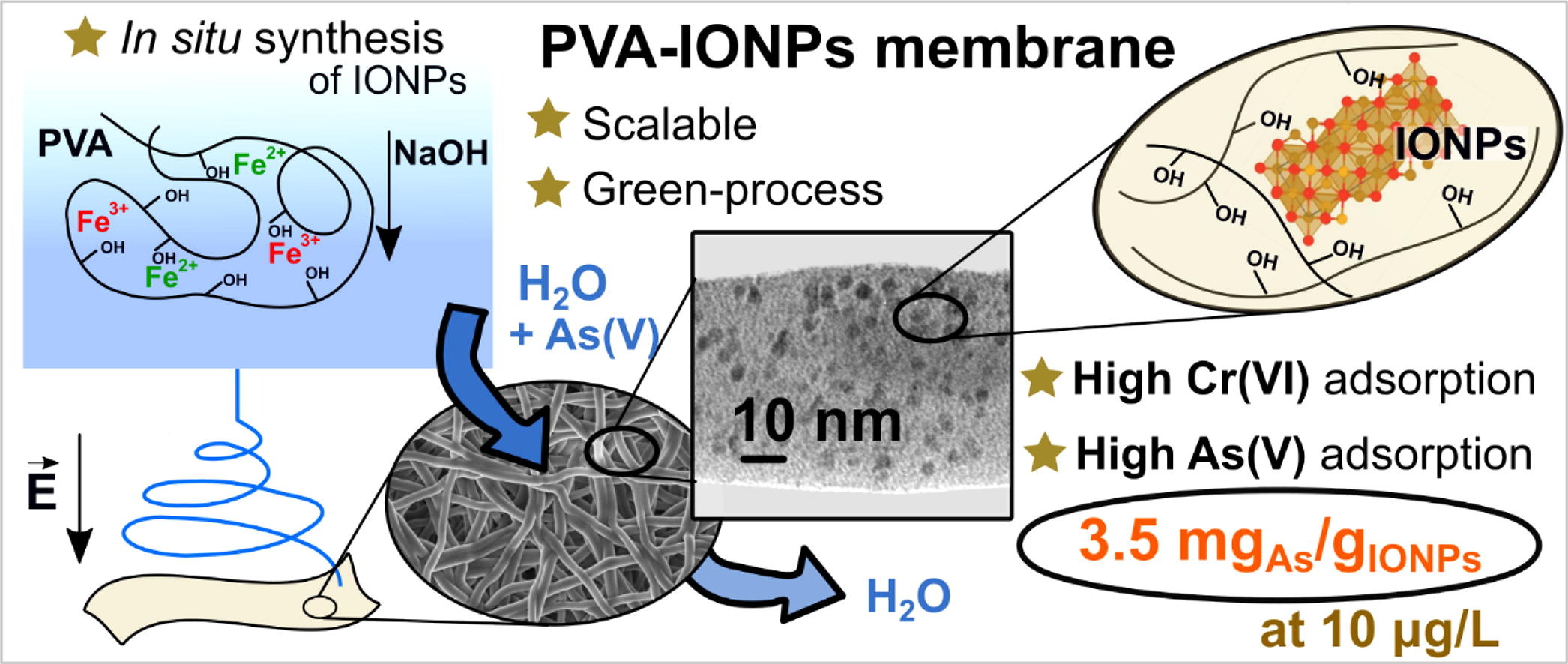
A team of researchers had successfully confined iron oxide nanoparticles in electrospun PVA nanofibres using the two-step procedure. The result was that the nanoparticles were excellently dispersed inside the nanofibres, but the quantity was too low (0.14 wt%). Now, the team shows 1 a novel approach to synthesizing in situ ultra-small iron oxide nanoparticles in electrospinning solutions.
The researchers obtained self-standing membranes with a considerable quantity of nanoparticles compared with other membranes in the literature. Due to excellent dispersion, tiny size, and surface availability of iron oxide nanoparticles, these membranes have high arsenic adsorption capacity and can reduce arsenic concentration below WHO threshold of 10 μg/L. Importantly, the researchers probed three reuse cycles with high removal effectiveness (84 %) and without migration of iron to the water.
The methodology to make these membranes can also be used for the adsorption of heavy metals such as Cr(VI) and Ni(II), with a remarkable adsorption capacity for Cr(VI) which, in addition, is independent of the Ni content.
More on the subject:
Drinking water using green moss
Converting photons from visible to UV to treat water
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Nicolás Torasso, Alicia Vergara-Rubio, Reinaldo Pereira, Javier Martinez-Sabando, José Roberto Vega Baudrit, Silvina Cerveny, Silvia Goyanes (2023) An in situ approach to entrap ultra-small iron oxide nanoparticles inside hydrophilic electrospun nanofibers with high arsenic adsorption Chemical Engineering Journal doi: 10.1016/j.cej.2022.140168 ↩