Polymorphism in non-fullerene acceptors for organic solar cells
Over the past several decades researchers have thought how materials and device architectures can efficiently convert solar radiation into electrical power through the photovoltaic effect. Those efforts have led to different methods and processes to produce green energy. In this context, organic photovoltaic (OPV) technology has received considerable attention because it indeed constitutes a new strategy for creating inexpensive solar cells that can be flexible and lightweight.
Conventional OPVs are made from an absorber or electron donating (D) material in conjunction with an electron accepting (A) material. Upon light absorption by the D material, an electron-hole pair, also known as exciton, is formed. Then excitons either relax to the ground state or migrate to the interface between the D/A heterojunction through exciton transfer processes. At the interface, free charge carriers (electrons and holes) are created and then collected.
Unlike most inorganic solar cells, where the exciton binding energy is negligible at room temperature, in OPVs the energy required to dissociate the excitons into charge carriers is substantial. Consequently, the efficiency of OPVs is much lower than that of traditional silicon cells. Nevertheless, the fact that OPVs can be mounted on lightweight, flexible, and easy-to-replace sheets, (which can be spread on roofs and buildings like wallpaper), makes this research field very attractive. Indeed, nowadays, more and more people in academy and industry are looking for new mechanisms to design organic solar cells (OSCs).
Most efficient OSCs include a blend of a D semiconducting polymer and an A semiconducting small molecule arranged in what is known as bulk heterojunctions (BHJs). Fullerene – a form of carbon where 60 carbon atoms are bonded together in a polyhedral structure composed of pentagons and hexagons informally called buckyball – derivatives have traditionally been the preferred choice for
the realization of BHJs. Recently, the use of so-called non-fullerene acceptors (NFAs) has atractted attention, mainly due to their stronger absorption in the visible and near-infrared regions and the surprisingly long exciton diffusion length.
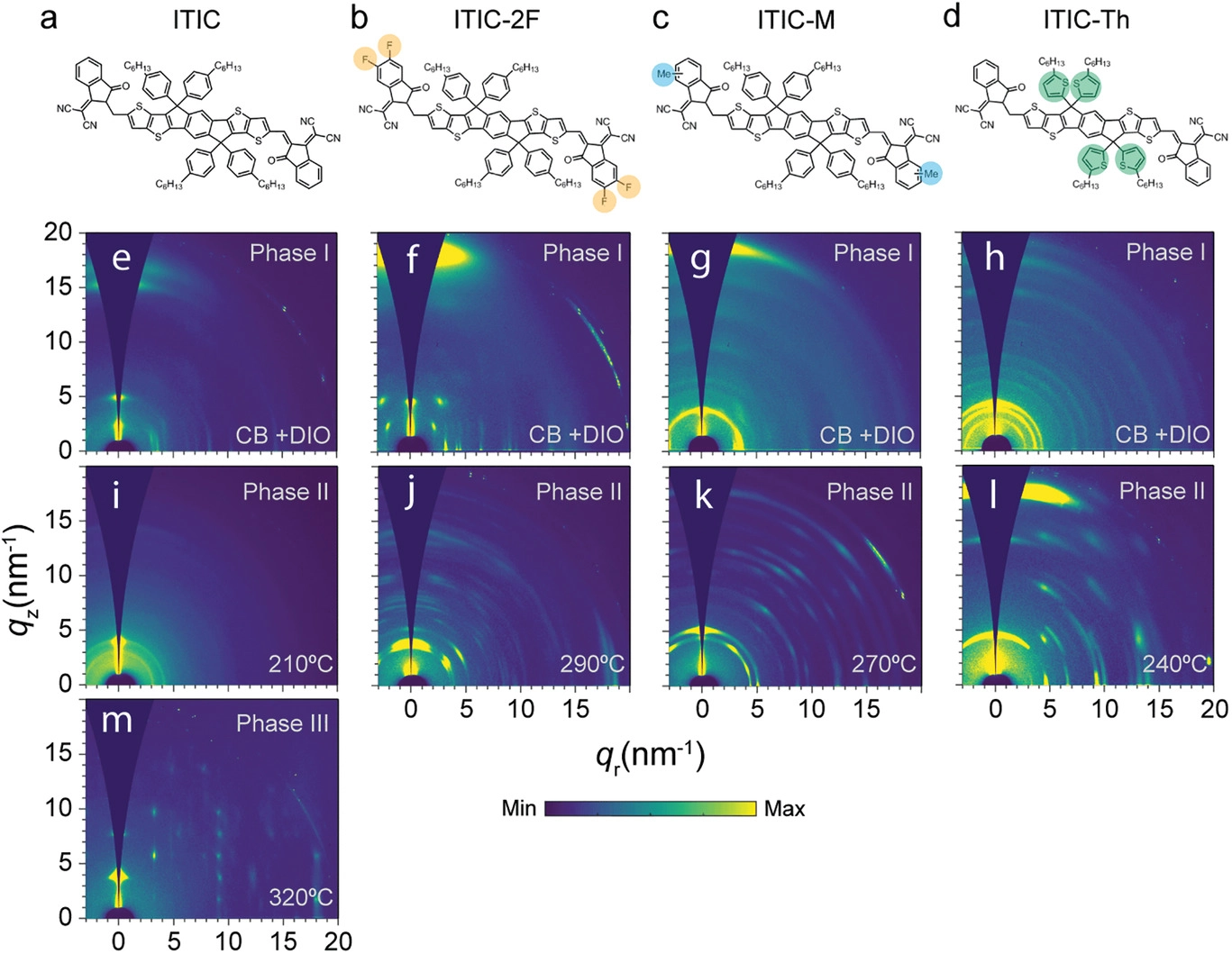
A highly successful example of a NFA is the indacenodith-ienothiophene-based molecule ITIC (chemical structure is shown in Figure 1a). Originally synthesized in 2015, ITIC is an A–D–A structured molecule with indacenodithieno[3,2-b]thiophene (IT) as the central D unit and with 2-(3-oxo-2,3-dihydroinden-1-ylidene)malononitrile (IC) as A end groups. While ITIC delivered initially a relatively modest power conversion efficiency, optimization of device processing, and incorporation of various chemical functionalities, such as fluorination (ITIC-2F, Figure 1b), methylation (ITIC-M, Figure 1c) or substitution of phenyl units by thiophene units (ITIC-Th, Figure 1d), led to an increase of the efficiency up to 14%.
Unlike fullerene derivatives, NFAs, including ITIC derivatives, often develop crystalline domains when they are solution-processed. This crystalline phase can adopt any of a variety of different crystal forms or polymorphs. Molecular polymorphism may affect device performance via different mechanisms: for example, different polymorphs can exhibit different optoelectronic properties or crystal habits might
differ between polymorphs, affecting the overall microstructure and leading to different BHJ phase morphologies. Thus, identifying the structural, optical, and electronic characteristics of the various crystalline phases NFAs can feature becomes a critical issue.
Now a team of researchers has characterized 1 the crystalline forms of ITIC-based NFAs, namely, ITIC, ITIC-M, ITIC-2F, and ITIC-Th. They find that at least two crystalline forms can be found in any of them.
The occurrence of polymorphism in organic materials, including organic semiconductors,can be understood in terms of the empirical Ostwald’s rule of stages, which states that in the crystallization of polymorphic systems, the most thermodynamically stable form is the last to appear. The polymorph initially formed is less thermodynamically stable but the activation energy required to overcome the barrier to form it is lower.
The researchers find that all four NFAs exhibit polymorphism, including a low-temperature metastable
polymorph that is characterized by multidimensional mesh-like aromatic structures and seemingly poor structural order along the π–π stacking direction; and a highly ordered thermodynamically stable high-temperature phase (or phases, in the case of ITIC). As packing motifs similar to the metastable polymorph have been reported for the best performing NFAs, this result suggests that these crystal forms are a common feature for the highest-performing electron acceptors for OSCs.
The new study reveals that the optical absorption of the structurally more disordered low-temperature phase can surpass that of the more ordered polymorphs while displaying comparable—or even higher—charge transport properties , providing a rationale for future materials discovery and crystal engineering.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Sara Marina, Alberto D. Scaccabarozzi, Edgar Gutierrez-Fernandez, Eduardo Solano, Aditi Khirbat, Laura Ciammaruchi, Amaia Iturrospe, Alex Balzer, Liyang Yu, Elena Gabirondo, Xavier Monnier, Haritz Sardon, Thomas D. Anthopoulos, Mario Caironi, Mariano Campoy-Quiles, Christian Müller, Daniele Cangialosi, Natalie Stingelin, and Jaime Martin (2021) Polymorphism in Non-Fullerene Acceptors Based on Indacenodithienothiophene Advanced Functional Materials doi: 10.1002/adfm.202103784 ↩