Quantum mechanics in biological systems (II): Gecko´s adhesion
The gecko, a climber´s hero, a lizard able to get a grip even face down on the smoothest ceiling or on the most vertical slippery surfaces. That, the adherence is one of the most striking features in most of the species in the family Gekkonidae. In Spain they are also known as “lagartijas” or “salamanquesas”. They are a good example beyond the trivial quantum effects, showing here quantum mechanics directly playing in the biological realm. Hence, entering the game of natural selection and evolution. The Gecko´s adhesion and the mechanism of their fascinating ability to hold its own weight with just a toe, like nailed to the most polished glass, has been matter of discussion during centuries. Many were the hypothesis for the amazing adherence system of this climber: suction, electrostatic attraction, micro-interlocking, and sticky secretions. Only two non excluding mechanisms are supported by evidence nowadays: capillary adhesion and intermolecular van der Waals attraction 1 Being at least the last one a direct quantum effect essential to get working such particular biological system.

In fact, the van der Waals attraction was shown in 2002 as the main cause of the mighty gecko´s grip2.Kellar Autumn and colleges subjected to exhaustive testing two competing hypotheses of adhesion mechanisms: thin-film capillary forces and van der Waals forces. Geckos lack secretory glands to excrete fluid required for adhesion by capillary, but a thin film of condensed atmospheric water could serve to this purpose. The capillarity is a process dependent on the surface tension which is caused by the cohesive forces in between liquid molecules. In water, the nature of these cohesive forces is varied. Being its main component the hydrogen bond, a consequence from the strong polarity of the water molecule. Water, as a polar molecule, generates capillarity forces which can act bringing together other hydrophilic (also polar) molecules or surfaces, always looking for the minimum energy state through any electrostatic interaction but not with the air. On the other hand, under the heading of van der Waals forces are grouped three particular weak forces meditating molecular attraction or repulsion. They occur between two permanent dipoles, between a permanent dipole and an induced dipole, and between two instantaneously induced dipoles (London dispersion force). The latter two types of van der Waals forces, (the last to a greater extent) are a direct consequence of quantum dynamics. They occur, regardless of the molecular symmetry, between non-polar molecules by fluctuating polarizations (instantaneous multipoles) derived from the movement of subatomic particles inside molecules and atoms .
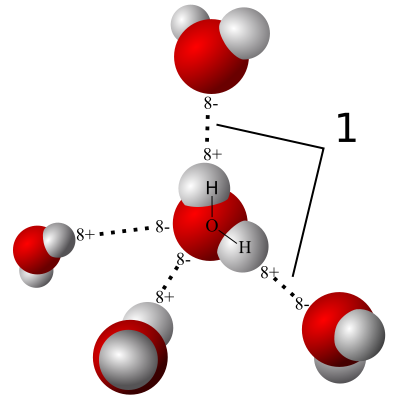
The reader uninitiated in the art could not have realized about the slight difference in between thin-capillary and van der Waals forces. In fact, the van der Waals forces are among the forces that mediate adhesion by capillary. The difference in between them is subtle, that is way it was so difficult to discern the root cause of this incredible adhesion mechanism. As we will see, the difference is subtle but the implications are enormous. In order to differentiate accurately, it was necessary to face the adhesive gecko´s footprint to surfaces able to modulate polarity and hydrophilicity. Using gallium arsenide (highly hydrophobic but highly polarizable semiconductor), hydrophilic or hydrophobic force sensors and silicon dioxide as a control (highly hydrophilic and polarizable), Autumn´s group was able to discern satisfactorily between both hypothesis. Ifcapillary forces dominate in the adhesion mechanism, a decreased adhesion would be shown on the strongly hydrophobic surfaces. Instead, if van der Waals forces have priority, nice adhesion should appear on the hydrophobic, but polarizable surfaces. In either case, there will be strong adhesion to the hydrophilic and polarizable control surfaces.
The outcome of those experiments pointed clear preponderance of van der Waals attraction forces driving gecko´s adhesion. Specially London dispersion force, because it is strong between polarizable (not meaning polar) surfaces and faintly dependent on the hydrophobicity. In addition, as we advanced above, capillary adhesion plays his role in this amazing mechanism. At this respect, again, Autumn and coworkers showed, although not a quantum effect, that the humidity affect the dynamic mechanical response and tensile properties of gecko setae (made of keratin), it increases the adhesion and friction. However, the extend of capillarity forces on this matter was measured for Tae Wan Kim and Bharat Bhushann, showing that hydrophilic surfaces increase geckos adhesion while increasing relative humidity. On the contrary, on hydrophobic surfaces, the adhesion decrease by an increase of the relative humidity3. Ergo in surfaces that repel water, the humidity makes geckos slip a bit, but in others, thim-film capillarity is a good gecko´s ally.
By now, we see that this awesome grip far from voodoo, is physics, quantum physics, but not only that. What is the secret behind a living system able to exploit the “magic” of quantum mechanics? How the weakness of van der Waals dispersion forces support that amazing grip? How those chubby and cushioned toes are able to stick without glue? Well, just because they hide in their palms a hierarchical structure made for increase the operative surface where those weak forces are able to act, allowing them add its insignificant strength to tie a giant. An evolution development arranged from visible lamellae scaling down through microscopic setae arrays containing millions of nanometric spatulae, which also are a mechanic self-cleaning system, always ready to grip (Figure 3).
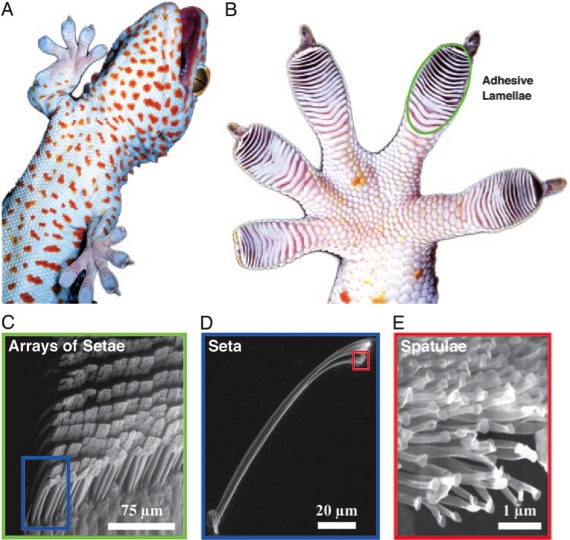
No glue, no hooks, no magnets, no electricity and what’s even better: no psychic fields or supernatural powers, only a smart evolved living system profiting quantum mechanic features to hunt and hide. Using dispersion forces instead of capillarity in the core of its adherence makes sure that he can rely on any surface. Remembering us an important lesson, from A.C.Clarke: “Any sufficiently advanced technology is indistinguishable from magic.”. Having understood the technology ceases to be magic, so anyone can use it. Imagine the possibilities of being able to reproduce the gecko adhesion, who wouldn´t want to look like his friend and neighbor spiderman…
References
- Prowse, M. S., Wilkinson, M., Puthoff, J. B., Mayer, G. & Autumn, K. Effects of humidity on the mechanical properties of gecko setae. Acta Biomater. 7, 733–738 (2011). ↩
- Autumn, K. et al. Evidence for van der Waals adhesion in gecko setae. Proc. Natl. Acad. Sci.99, 12252–12256 (2002). ↩
- Kim, T. W. & Bhushan, B. The adhesion model considering capillarity for gecko attachment system. J. R. Soc. Interface 5, 319–327 (2008). ↩
4 comments
[…] Quantum mechanics in biological systems (II): Gecko´s adhesion […]
Above studies are all of my interest and I am looking forward to read in the future.
[…] gora edo buruz behera ibiltzen den geko muskerraren oinetan topatu zuen inspirazioa etorkizun oparoko beste itsasgarri batek. Gekoak […]
[…] jasoko lukete albistea: haien eztabaida ebatzita dago. Gekoak iguanak baino lehenago agertu ziren. Kito. Hori adierazten du, bederen, paleontologo talde batek […]