Do cation-cation and anion-anion attractive interactions exist?
The pairing of ions of opposite charge is one of the dogmas in chemistry and ion-pairs or ionic interactions often govern chemical reactions or more particularly such processes as synthesis or catalysis. Usually, there is no doubt that ions possess opposite charges. This is why the recent report on the existence of cation-cation pairing by hydrogen bond interactions in crystal structures of 4-oxopiperidinum salts seems to be surprising1. It is worth to mention that few experimental and theoretical studies on interactions between ions of similar charge were published during the last several years, but they could arouse controversies. The recent experimental study seems to prove conclusively that separated cations not supported by interactions with anions are linked by stabilizing interactions.
To describe the recent study let us start from the history of this topic. In 1998 Braga and co-workers discussed the crystal structure of potassium hydrogen oxalate, KHC2O42, where the arrangement of ions in crystal shows HC2O4– anion-anion links through hydrogen bonds in spite of the delocalization of negative charge over the whole anions. The authors have calculated the interaction energy for two HC2O4– units linked by the O-H…O hydrogen bond with the geometry taken from the crystal structure; this energy is positive and amounts +39 and +46 kcal/mol for HF and DFT(B3LYP) methods, respectively, thus the pairing process is not energetically favourable. The electrostatic potential calculated for HC2O4– anion is negative for the whole molecular surface. This is why Braga and co-workers have concluded that the stability of the anionic chain (see Fig. 1) arises from the presence of K+ cations and that K+…HC2O4– attractive interactions predominate over repulsive K+…K+ and HC2O4–… HC2O4– ones. Finally, they remarked that the O-H…O interactions in the anionic chain may be classified as pseudo hydrogen bonds where the repulsions between anions are minimized, but those interactions are not stabilizing ones.
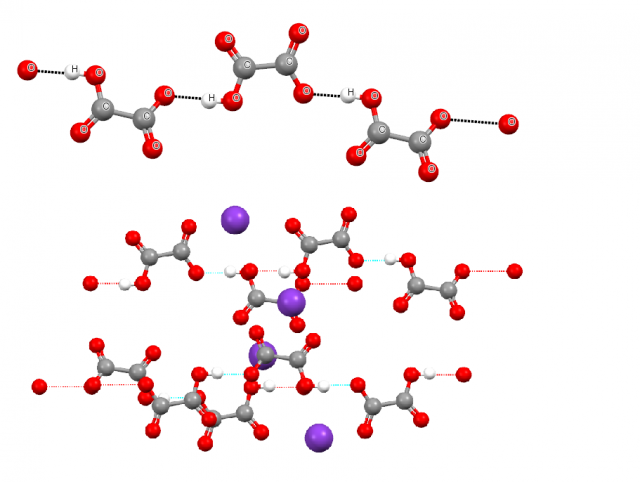
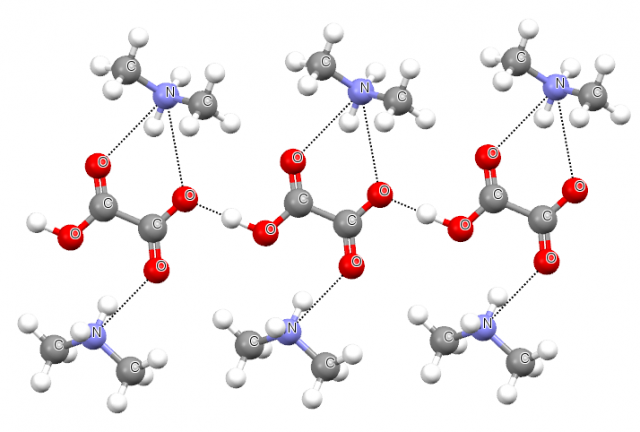
Steiner also has analyzed the crystal structure of potassium hydrogen oxalate3, he criticized the described above study and he concluded that the experimental results on this structure indicate that the O-H…O interactions between anions do not differ fundamentally from classical hydrogen bonds. The O-H proton donating covalent bond as well as the oxygen proton acceptor within O-H…O system possesses features typical for strong hydrogen bonds. Steiner also analyzed the crystal structure of dimethylammonium hydrogen oxalate where similar anion-anion chains linked through O-H…O hydrogen bonds are observed and where those chains may be stabilized by the additional N-H…O hydrogen bonds between hydroxalate anions and dimethylammonium cations [4]. Figure 2 presents the fragment of the latter structure where the mentioned above interactions are indicated.
The other study supports the statements of Steiner on the nature of the mentioned above O-H…O interactions [5]. The authors performed extensive X-ray (T = 11 K) and neutron (T = 15 K) diffraction measurements on crystals of potassium hydrogen oxalate. It was confirmed here that the experimental geometrical parameters of the O-H…O connections are well attributed to typical strong hydrogen bonds since the O-H bonds in O-H…O links are elongated if compared with isolated HC2O4– hydroxalate anion. The short H…O distance of 1.457 Å observed for this structure is also typical for strong hydrogen bonds possessing the covalent character.
However the most important is that the analysis of the experimental electron charge density was performed for the potassium hydrogen oxalate structure4 (the pseudomolecule approach based on the atomic multipolar expansions56 was used to reproduce the charge density through reciprocal space fitting of structure factors derived from low-temperature X-ray diffraction data). It was found that a bond path and characteristics of a corresponding bond critical point for the H…O contacts indicate the strong stabilizing hydrogen bond interactions [5]. Besides the experimentally determined electrostatic potential for the HC2O4– anion in the crystal structure [5] is quite different than the potential for isolated anion calculated theoretically. For the latter case, as it was reported by Braga and co-workers [2], the whole ion surface is characterized by the negative electrostatic potential. In the crystal, in the vicinity of hydrogen atom the negative lobe is absent and at the position of the proton acceptor O-atom the electrostatic potential is positive [5]. The latter observations are in contrary to the statement that for the HC2O4– anionic chains the repulsive electrostatic interactions exist [2].
Summarizing, one can see from those early studies on interactions between ions of like charges that the interactions between anions are possible due to the links which possess characteristics of strong hydrogen bonds. Additionally the latter interactions do not differ fundamentally from the other classical hydrogen bonds. However, one important question arises: how other interactions, especially those between anionic chains and cations ¨gluing¨ the crystal structure, influence on properties of the mentioned above hydrogen bonds? It seems that those additional interactions with cations influence on the distribution of the electron charge density for anionic chains causing that the features of anion-anion links are well fitted to the typical hydrogen bonds. Note that the stable and isolated (especially isolated from the interactions with cations) dianions linked through hydrogen bonds are not observed experimentally. This is why the topic described here came back in few recent publications.
The F–…HCO3– anion-anion complex was analyzed theoretically7 and it was found that the complex formation is an endothermic process since its energy is higher than for the isolated anions by +56.8 kcal/mol (G = +63.4 kcal/mol). However, this complex is situated at the shallow local minimum of about ~0.1 kcal/mol [8]. This study was rightly criticized8 because of the methodology of calculations applied, also the 0.1 kcal/mol value mentioned above is within the error of calculations thus it is not important (does not confirm any energetic minimum).However, the concept of stable interactions between like-charge ions is not doomed to fail. Recent studies of Alkorta and co-workers should be mentioned here since numerous anion-anion complexes joined by hydrogen bonds were analyzed theoretically and local energy minima were found for them in spite of electrostatic repulsions91011. The latter studies show the crucial role of environment for the stabilization of the anion-anion interactions.
The recent experimental study of Pörschke and co-workers [1] seems to be very important; the series of crystal structures of 4-oxopiperidinium salts, [OC5H8NH2]X (different X anions were considered), was analyzed; for numerous cases the cation-anion chains linked through the well known charge-assisted hydrogen bonds are observed. For the crystal structure of [OC5H8NH2][N(SO2CF3)2] the cationic chain linked through hydrogen bonds is observed; i.e. cation-cation N-H…O hydrogen bonds are detected for the latter structure. This chain is stabilized by the additional interactions with [N(SO2CF3)2]– anions. This is similar to the crystal structure of potassium hydrogen oxalate discussed earlier here, where the anionic chain is stabilized by additional interactions with cations.
![Figure 3. The fragment of the crystal structure of [OC5H8NH2][AlOC(CF3)34] 2(C2H5)2O, (upper diagram) dication situated in the central part of this picture is surrounded by neutral (C2H5)2O molecules, while anions are far from dication, (lower diagram) the structure of dication is presented, it is stabilized by the C-H…O hydrogen bonds, O, C, H and N atoms are designated by red, grey, bright grey and blue colors, respectively; pink and green colors for atoms in anions correspond to aluminum and fluorine, pictures taken from Cambridge Structural Database (CSD) | Credit: F.H.Allen, The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Cryst. 2002, B58, 380-388.](https://mappingignorance.org/app/uploads/2015/09/cation-3-446x640.png)
However interesting and unexpected arrangement of molecules is observed for the [OC5H8NH2][Al{OC(CF3)3}4]2(C2H5)2O crystal structure [1]. The [OC5H8NH2]22+ dication is linked here by the C-H…O hydrogen bonds. It seems that such dications in the crystal structure are not stabilized by interactions with [Al{OC(CF3)3}4]– anions since these dications are in contact with the neutral (C2H5)2O molecules while anions are far from them (see Fig. 3). The authors note in the article [1]:
¨In the tetra(perfluoro-tert-butoxy)aluminate salt, the anions are fully separated from the cations, and the cations associate pairwise by N-C-H···O=C hydrogen bonds. The compounds represent the first genuine examples of self-association of simple organic cations based merely on hydrogen bonding as evidenced by X-ray structure analysis, and provide a paradigm for an extension of this class of compounds.¨
References
- W.Gamrad, A.Dreier, R.Goddard, K-R.Pörschke, Cation-Cation Pairing by N-C-H…O Hydrogen Bonds. Angew. Chem. Int. Ed. 2015, 54, 4482-4487. ↩
- D.Braga,F.Grepioni,J.J.Novoa, Inter-anion O–H–···O–hydrogen bond like interactions: the breakdown of the strength–length analogy. Chem.Comm. 1998, 1959-1960. ↩
- T.Steiner, Inter-anion O–H···O interactions are classical hydrogen bonds. Chem.Comm. 1999, 2299-2300. ↩
- P.Macchi, B.B.Iverson, A.Sironi, B.C.Chakoumakos, F.K.Larsen, Interanionic O-H…O Interactions: The Charge Density Point of View. Angew. Chem. Int. Ed. 2000, 39, 2719-2722. ↩
- V.G.Tsirelson, R.P.Ozerov, Electron Density and Bonding in Crystals.Bristol, Philadelphia; Institute of Physics, 1996. ↩
- P.Coppens, X-Ray Charge Densities and Chemical Bonding. IUCr, Oxford University Press, 1997. ↩
- F.Weinhold, R.A.Klein, Anti-Electrostatic Hydrogen Bonds. Angew. Chem. Int. Ed. 2014, 53,11214-11217. ↩
- G.Frenking, G.F.Camamori, No Need for a Re-examination of the Electrostatic Notation of the Hydrogen Bonding: A Comment. Angew. Chem. Int. Ed. 2015, 54, 2596-2599. ↩
- I.Mata, I.Alkorta, E.Molins, E.Espinosa, Electrostatics at the Origin of the Stability of Phosphate-Phosphate Complexes Locked by Hydrogen Bonds. ChemPhysChem 2012, 13, 1421-1424. ↩
- I.Mata, I.Alkorta, E.Molins, E.Espinosa, Tracing environment effects that influence the stability of anion–anion complexes: The case of phosphate–phosphate interactions. Chem.Phys.Lett. 2013, 555, 106-109. ↩
- I.Mata, E.Molins, I.Alkorta, E.Espinosa, The Paradox of Hydrogen-Bonded Anion−Anion Aggregates in Oxoanions: A Fundamental Electrostatic Problem Explained in Terms of Electrophilic···Nucleophilic Interactions. J.Phys.Chem. A 2015, 119, 183-194. ↩
1 comment
[…] Las cargas positivas se repelen entre sí, y las negativas lo mismo. ¿Siempre? Slawomir Grabowski, del DIPC, nos adentra en este misterio en Do cation-cation and anion-anion attractive interactions exist? […]