The chemical bond – is this term still useful or should it be replaced by another one?
¨The chemical bond¨ is one of the most often used concepts in chemistry. It is also one of the eldest terms since the first definition of chemical bond comes from the studies of G.N. Lewis who published his classical paper on interactions between atoms in 1916 1. Lewis stated that ¨ we shall say that there is a chemical bond between two atoms or groups of atoms in case that the forces acting between them are such as to lead to the formation of an aggregate with sufficient stability to make it convenient for the chemist to consider it as an independent molecular species.¨ This is strange, but one would think that nothing has changed since then. For example, in Compendium of Chemical Terminology Gold Book (edition of 2014!) 2 the International Union of Pure and Applied Chemistry recommends the definition of the chemical bond which is word for word the same as that one given by Lewis! Besides one may ask if this definition is useful at all, for someone accustomed to precision such determinations as ¨sufficient stability¨ or ¨convenient… to consider it¨ are unacceptable.
Of course, it does not depreciate the valuable research of Lewis who also stated that ¨the chemical bond is at all times and in all molecules merely a pair of electrons held jointly by two atoms.¨ It seems that the concept of the pair of electrons shared by two atoms is used by most chemists (at least by those who are not involved in advanced theoretical research) since it is applied not only in elementary chemistry courses but also in advanced ones; also other concepts of Lewis, such as ¨dots structures¨ (where dot designate an electron) known also as Lewis structures, octet rule or Lewis acids and bases are commonly applied. The concept of shared pair of electrons or the term ¨Lewis structure¨ are very useful in numerous problems in spite of the fact that they are often far from the reality.
The studies on chemical bond were continued by others, especially by Pauling who introduced such terms as covalent bond, metallic bond or ionic bond 3. Briefly speaking (what is often stated in chemistry books, even often in those for students) the covalent bond corresponds to the shared pair of electrons between the same atoms; if atoms are not the same this pair is moved towards the more electronegative atom (polarized bond) and in the extreme case, if atoms differ significantly in electronegativity the electron pair is located almost completely with a more electronegative atom; it is a case of the ionic bond.
This is a very simplified picture, but I do not like to explore issues using advanced theoretical chemistry. A very detailed and comprehensive analysis was performed recently 4. It is worth mentioning for purposes of this article that the application of the Lewis-Pauling concepts encounters many difficulties in numerous cases [4]. The one-electron and three-electron bonds are analyzed in different theoretical and experimental studies Another problem concerns the formation of chemical bond – which interaction may be classified as a bond and which yet is not? For example, the fluorine ion, F–, interacts with HF molecule forming the linear [FHF]– ion where the hydrogen is situated in the mid-point of the F….F distance. The H…F contacts in this ion are very short, like in covalent bonds, and the corresponding H…F interactions are also strong enough to be classified as covalent bonds. This problem was analyzed early on by Pauling and the divalency of hydrogen in [FHF]– anion was considered [3]. However, the H-atom could not be divalent since it is in contrast to all commonly accepted rules (among them those of Lewis and Pauling).
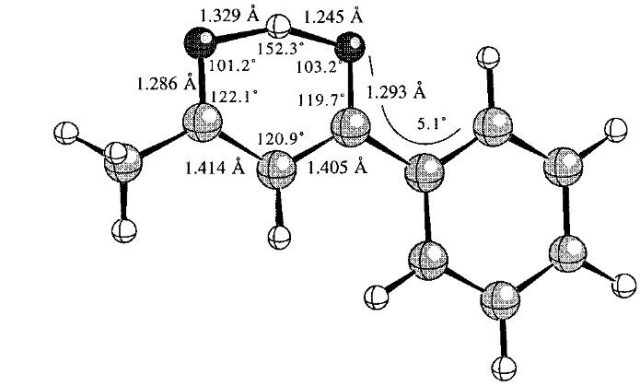
The arrangement of atoms in the [FHF]– ion may be treated as the hydrogen bond interaction [3]; the same problem as for the latter ion arises for numerous very strong hydrogen bonds which possess numerous characteristics of typical covalent bonds 5. For example, Fig. 1 presents the experimental molecular structure of benzoylacetone taken from the crystal structure solved by the neutron diffraction technique 6. One can see here two H…O distances (1.245 and 1.329 Å); it is difficult to classify one of them (shorter) as the covalent bond and the second one (longer) as the intramolecular attractive interaction since both are relatively short and both may be classified as covalent bonds. There are also two 1.286 and 1.293 Å C-O bonds (single or double?) and two C-C bond lengths equal to 1.405 and 1.414 Å (single or double?) in this structure. This structure can not be expressed by a single Lewis formula since the strong electron delocalization occurs here. This is true, one may consider this molecular structure as the combination of two resonance structures (Lewis structures) but it is a case when the real structure (detected experimentally) is expressed by not existing ones where the valence electrons are well located. There are other numerous examples of molecular structures where the commonly accepted rules and terms (among them ¨the chemical bond¨) can not be directly applied.
It seems that the QTAIM (Quantum Theory of Atoms in Molecules) approach7 introduced by Richard Bader is a useful tool to analyze interatomic interactions. QTAIM allows to analyze electron charge distribution of different systems (atoms, ions, molecules, complexes, up to great aggregates, i.e. clusters and crystals). It is applied for the analysis of the experimental electron density or the electron density calculated by different theoretical techniques 8. This is important that all terms which were introduced within the QTAIM approach are strictly connected with the electron charge distribution and they are rooted in precise mathematical formulas; thus one does not have such doubts as in a case of the definition of the chemical bond presented earlier here.
The local maxima of electron density correspond to the positions of nuclei. There are other special points of the considered electron density; these are critical points for which the gradient of electron density vanishes. In other words, the critical points may correspond to the maxima, to the saddle points or to the minima of the electron density. The bond critical point (BCP) corresponds to the pair of interacting atoms and it is the minimum of electron density on the bond path. For the structure in energetic minimum, the bond path (BP) is the line of maximum electron density connecting two interacting atoms. Hence, two atoms are connected by the bond path if the stabilizing interaction occurs between them and it does not matter if it is the weak van der Waals interaction or the chemical bond in the classical sense. It is important that BP, BCP, and the stabilizing interaction are precisely expressed by the mathematical equations. The system of atoms (or ions) linked by bond paths with the locations of critical points is usually named as the molecular graph. The laplacian of the electron density is another important term in QTAIM approach since it informs of the concentration or of the depletion of the electron charge if it is negative or positive, respectively.
The properties of the specified interaction between atoms detected by the existence of the bond path may be expressed by characteristics of the corresponding bond critical point. These characteristics are often related to old and commonly known in chemistry terms such as covalent character, ionicity, etc. Hence for shared interactions like covalent and polarized bonds the laplacian of electron density is negative since there is a concentration of electron density in the atom-atom region. For interactions between closed-shell systems like van der Waals interactions, ionic ones and weak and medium in strength hydrogen bonds there is the depletion of electron charge within the atom-atom region thus the laplacian is positive here.
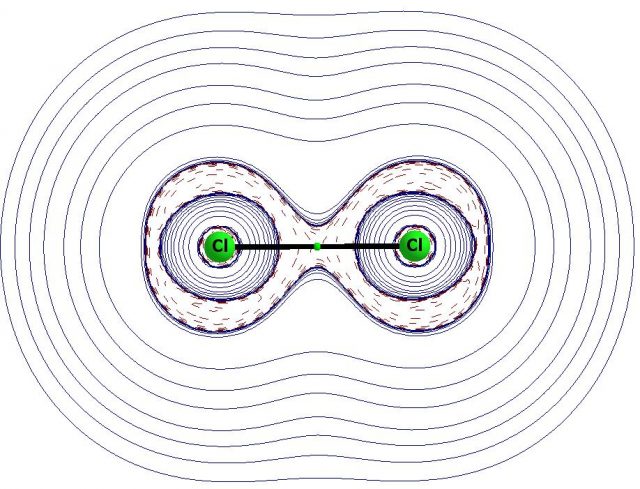
Fig. 2 presents the molecular graph of the chlorine molecule with the bond path linking two Cl-atoms. The bond critical point is situated on the bond path and it belongs to the interatomic region of the negative laplacian of the electron density. Hence the Cl-Cl link may be considered as the covalent bond. The regions of negative laplacian, i.e. the regions of the concentration of the electron density around the Cl-atoms correspond to the electron shells. This is a simple example but one can see here a clear situation, the existence of the bond path informs of the stabilizing interaction and the characteristics of the corresponding bond critical point belonging to this path inform of the character of interaction (not only the laplacian of electron density is usually analyzed by the QTAIM approach but also other characteristics not mentioned in this text).
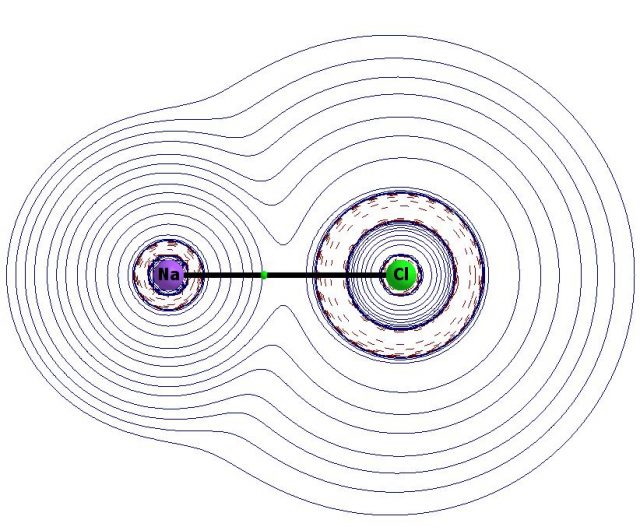
Fig. 3 presents the molecular graph of the NaCl species which is traditionally considered as a case of the ionic bond. One can see the bond path linking Na and Cl atoms. The BCP lying on this path is characterized by the positive value of the laplacian of electron density (BCP is situated in the region of positive laplacian – Fig. 3). It means that the Na-Cl link is a stabilizing interaction classified as the ionic bond between two closed-shell systems (Na+ and Cl– ions). There are spheres of the negative laplacian around the sodium and chlorine ions; the sodium ion is positive since the Na-atom looses electron (one negative laplacian value ring is observed for Na+); the chlorine ion is negative since the Cl-atom withdraws an electron (two rings of negative laplacian are observed here – it means L and M electron shells; the K-shell is not visible since it is thin and it is situated within the Cl ion designation).
Let us look at the mentioned above case of the [FHF]– anion. Fig. 4 shows the molecular graph of this system. One can see here two equivalent H…F bond paths with the symmetrically located BCPs. Both bond critical points lie in the region of the negative laplacian thus both H…F links may be considered as covalent bonds. However one should note that the term covalent bond is strongly related to the Lewis structures. The classification of interactions based on a sign of laplacian of electron density at BCP corresponding to the bond path is only related to such concepts as covalent bond, ionic bond but the latter terms are not derived from the QTAIM approach. In other words one can not say that the results of QTAIM approach confirm the divalent character of the H-atom in the [FHF]– ion.
![Figure 4. The molecular graph of the [FHF]- species, the solid straight line corresponds to the bond path, the big circles to atoms and the small green circle to BCP, the isolines of laplacian of electron density are also presented, the positive values of laplacian are depicted in the solid lines and the negative values in the broken lines.](https://mappingignorance.org/app/uploads/2015/08/Bond-4-640x430.jpg)
Fig. 4 shows the regions of negative laplacian around F-atoms and also around H-atom. The latter seems to be strange. However one should note that the formation of the [FHF]– ion leads to the electron charge redistribution, the electron charge is moved from F-atoms to the central H-atom. It is not a situation where two pure F– ions are connected with a pure proton, H+. The negative laplacian values at the H….F BCPs inform of the electron density shifts from F-centres to the hydrogen centre. The MP2/aug-cc-pVTZ calculations show that the QTAIM charge calculated at the H-atom in the [FHF]– ion is equal to +0.75 au (not +1 au!).
One can see that the term ¨bond path¨ covers cases of atom-atom energetically stabilized links, from weak van der Waals interactions, through stronger Lewis acid – Lewis base interactions up to covalent bonds. The location of bond paths also allows interpreting mechanisms of interactions and, in general, of chemical reactions 9. Hence the question arises if this term is more useful than the old-fashioned ¨chemical bond¨ which is not well defined. The bond path can describe a complete range of bonded interactions and it is attributed to the electron charge distribution between the pair of atoms. On the other side, the chemical bond is limited and dominated by the pair-electron concept of Lewis. Thus it seems that the term ¨bond path¨ at least should be considered as an alternative way to describe interactions within systems analyzed.
Richard Bader who introduced the QTAIM approach writes in one of his articles 10:
¨Thus the notion of a chemical bond is too restrictive and is ill-suited to account for the physics underlying the spectrum of interactions between atoms and molecules that determine the properties of matter. What is needed is a definition of bonding rather than of a bond, one that can describe the complete range of bonded interactions that account for the properties of all matter. Such is the role fulfilled by a bond path.¨
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- ¨The Atom and the Molecule¨. G.N.Lewis, J.Am.Chem.Soc. 1916, 38, 762-785. ↩
- ¨Compendium of Chemical Terminology Gold Book¨. International Union of Pure and Applied Chemistry, 2014, Version 2.3.3 ↩
- ¨The Nature of the Chemical Bond¨. L. Pauling, Third Ed. Cornell University Press, Ithaca, New York, 1960. ↩
- ¨The Chemical Bond: Fundamental Aspects of Chemical Bonding¨. Eds. G.Frenking and S.Shaik, Wiley-VCH, Weinheim, Germany, 2014. ↩
- ¨What Is the Covalency of Hydrogen Bonding?¨ S.J.Grabowski, Chem.Rev. 2011, 11, 2597-2625. ↩
- ¨Characterization of the Short Strong Hydrogen Bond in Benzoylacetone by ab Initio Calculations and Accurate Diffraction Experiments. Implications for the Electronic Nature of Low-Barrier Hydrogen Bonds in Enzymatic Reactions¨. B.Schiøtt, B.B.Iversen, G.K.H.Madsen, T.C.Bruice, J.Am.Chem.Soc. 1998, 120, 12117-12124. ↩
- ¨Atoms in Molecules, A Quantum Theory¨. R.F.W.Bader, Oxford University Press, Oxford, 1990. ↩
- ¨Electron Density and Bonding in Crystals¨. V.G.Tsirelson, R.P.Ozerov, Bristol, Philadelphia; Institute of Physics, 1996. ↩
- ¨What can be learnt from a location of bond paths and from electron density distribution¨. S.J.Grabowski, Chapter in the book: ¨Applications of Topological Methods in Molecular Chemistry¨. Eds. R. Chauvin, C.Lepetit, B.Silvi, E.Alikhani, Series: Challenges and Advances in Computational Chemistry and Physics, Springer International Publishing AG, Cham, in press. ↩
- ¨Bond Paths are not Chemical Bonds¨. R.F.W.Bader, J. Phys. Chem. A 2009, 113,10391–10396. ↩
1 comment
[…] Desde muy temprano en el estudio de la química nos habituamos el término “enlace químico”. Pero, ¿tiene sentido seguir hablando de enlace químico para referirnos a las interacciones entre átomos tras lo que hemos aprendido en las últimas décadas? […]