Strange molecular systems in the atmosphere of Titan
Imagine there is another world in the universe similar to the Earth, where there are mountains, rivers and seas. There are also winds, volcanoes, clouds and rains… science fiction, no, such a world does exist and not too far from the Earth if one considers the scale of the universe. This is the largest moon of the planet Saturn – Titan.
There is the rapid increase of scientific articles as well as news related to Saturn´s moon Titan in recent years. For example the latest news from October 24, 2014 – NASA Finds Methane Ice Cloud in Titan’s Stratosphere or from September 29, 2014 – Cassini Watches Mysterious Feature Evolve in Titan Sea. Why there are so extensive investigations of this satellite of the far planet Saturn (far if one relates to the Earth scale)? It is worth to mention that the observations of Titan are possible thanks to the Cassini-Huygens unmanned spacecraft (Figure 1) sent to Saturn; this spacecraft started to study Saturn and its numerous satellites since arriving there in 2004; about 60 Saturn´s moons are known till now with confirmed orbits but only 13 of them have diameters larger than 50 kilometers.

Titan holds fascination of investigators since it is the only moon in the solar system with an atmosphere similar to Earth´s one, atmosphere made mostly of N2 possesses a rich organic chemistry thanks to the second most abundant constituent, methane (about 1.4% in the stratosphere).Titan’s atmosphere is like a chemical factory since it initializes the formation of various ions; numerous original chemical species are formed in the high thermosphere as a consequence of magnetospheric, ionospheric and atmospheric interactions involving solar radiation, electrons and energetic ions. The results collected by the Cassini instruments suggest that the chemical reactions produce large organic molecules such as benzene, naphthalene, etc.
Titan is the largest Saturn’s moon which displays chains of mountains, fields of dark and damp dunes, lakes and possibly geologic activity. This moon is larger than the Earth´s moon, for comparison see Figure 2. The thick atmosphere of Titan is orange due to a dense organonitrogen haze.

The investigations on Titan are fascinating; hundreds of articles concerning different aspects of this moon are published every year. This satellite is often compared with the Earth since it possesses similar characteristics, wind, rain, volcanoes and other features are similar; the organic chemistry reactions occurring in the Titan atmosphere are analogous to those which took place in the early atmosphere of the Earth. This is the reason of numerous speculations if there are any forms of life on Titan or if they will occur in the future.
However the mean temperature of Titan is of about -180oC and the surface pressure is higher (1.6 bar – Titan, 1 bar – Earth) what results in numerous exciting differences between these two bodies. Clouds and rains of methane and ethane occur on Titan, similarly rivers, lakes and seas of liquid methane and ethane are characteristic for this moon. The greatest Titan´s sea – Ligeia Mare (Ligeia is one of the Sirens in Greek mythology), 420 km across, is comparable to Lake Superior on Earth (see Figure 3).

It is important that numerous chemical processes are catalyzed in the atmosphere of Titan due to the solar radiation. For example, the reactions of different carbocations with neutral hydrocarbons are analyzed 1; like the reaction of vinyl cation with acetylene 2. The methyl cyclopropane cation is the most energetically stable product of the latter reaction (Figure 4) but the other products correspond to the local energetic minima. Among them there is an interesting complex where the proton is situated between two perpendicular acetylene molecules34. Figure 5 presents the molecular graph of the latter species. What is unique for this complex? The proton is closer to one of acetylene molecules since the distances between the proton and the middles of CC acetylene bonds are equal to 1.31 and 1.67 Å, these distances are designated as π-H and π…H, respectively hereafter (MP2/aug-cc-pVDZ results of calculations are presented here and afterwards in this text). This is why this complex may be treated as an interaction between the C2H3+ cation and the C2H2 molecule. In such a way the binding energy of the complex related to the C2H3+ and C2H2 species amounts -15 kcal/mol. This is the stronger interaction than the hydrogen bond for the most stable configuration of the water dimer where the binding energy is equal to -5 kcal/mol – slight differences are observed for different levels of calculations.

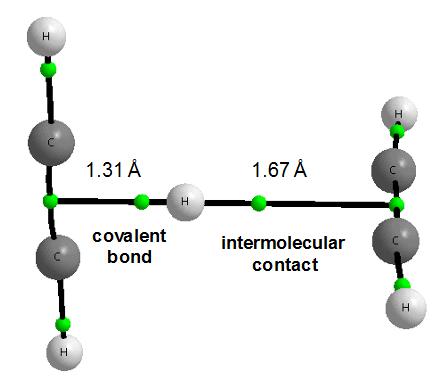
There are interesting consequences from the asymmetric position of the proton between the acetylene molecules. The consideration of the C2H3+ cation as an integrated unit seems to be justified since the π-H contact of 1.31 Å possesses the characteristics of the typical covalent bond. This is an unusual situation since the covalent bonds are traditionally related to the links between two atoms. For the isolated C2H3+ cation as well as, consequently, for this cation within the C4H5+ species (see Figure 5) we have the connection between the proton and the CC bond thus it is the case of three-center covalent bond.
The Quantum Theory of Atoms in Molecules (QTAIM) 5 may clear up this situation since within QTAIM approach the bond paths are specified as the lines linking the local maxima of the electron density (usually attributed to the positions of atoms). There is the bond critical point assigned to each bond path. For acetylene molecules in the C4H5+ complex one can observe in Fig. 5 the bond paths (solid black lines) corresponding to the C-H and CC bonds and each of them is characterized by the corresponding bond critical point. The similar bond paths are observed not only for typical covalent bonds but also for intermolecular atom-atom contacts; it was stated that the bond paths correspond to preferable interactions 67. The bond path usually corresponds to the pair of atoms since it is the atom-atom connection with the corresponding bond critical point; in general bond paths correspond to the covalent bonds or to the intermolecular contacts.
However an untypical situation is observed for the C4H5+ complex where the central proton is linked through bond paths with bond critical points of acetylene molecules (Figure 5), two such connections correspond to the π-H and π…H contacts described above. One can say that the bond critical points related to the CC bonds mimic here the single centers. On the other hand the characteristics of the bond critical points of the π-H and π…H contacts may be used to describe the nature of the corresponding interactions. The analysis of those characteristics led to the conclusion that the π-H contact possesses the features characteristic for the covalent bond while the π…H features indicate the typical intermolecular interaction.
It is interesting that for the C4H5+ complex the proton situated in the central part of the system is not exactly the particle characterized by the +1 au charge. Its charge amounts +0.4 au what is a consequence of strong interactions with acetylene molecules, especially with the closer one. These interactions lead to the significant electron charge shifts decreasing the charge of the central proton (maybe the term ¨hydrogen atom¨ is more accurate here).
One can see that the investigations of Titan are exciting not only because of interesting geology, meteorology or chemical reactions on this moon, similar to those occurring on the Earth but also because of the chemical systems which may exist in Titan´s atmosphere. These chemical systems show new, unexpected properties which have not been known before.
References
- M.Larsson, W.D.Geppert, G.Nyman, Ion chemistry in space, Rep. Prog. Phys. 75 (2012) 066901 (75pp) ↩
- G. E. Douberly, A. M. Ricks, B. W. Ticknor, W. C. McKee, P. v. R. Schleyer, M. A. Duncan, Infrared Photodissociation Spectroscopy of Protonated Acetylene and Its Clusters,J. Phys. Chem. A 112 (2008) 1897-1906. ↩
- S.J.Grabowski, Dihydrogen bond and X-H… interaction as sub-classes of hydrogen bond, J.Phys.Org.Chem. 26 (2013) 452-459. ↩
- S.J.Grabowski, W.A.Sokalski, J.Leszczynski, Is a π…H+…π Complex Hydrogen Bonded, 108 (2004) 1804-1812. ↩
- R. F. W. Bader, Atoms in Molecules, A Quantum Theory; Oxford University Press, Oxford, 1990. ↩
- R.F.W.Bader, Bond Paths Are Not Chemical Bonds, J.Phys.Chem. A 113 (2009) 10391-10396. ↩
- S.J.Grabowski, J.M.Ugalde, Bond Paths Show Preferable Interactions: Ab Initio and QTAIM Studies on the X-H…π Hydrogen Bond, J.Phys.Chem. A 114 (2010) 7223-7229. ↩