Is GABA a link between gut and brain?
Is GABA a link between gut and brain?
The human microbiota is the community of microorganisms that resides in our body. They are found in a number of organs and biofluids, including the skin, mammary glands, placenta, seminal fluid, uterus, ovarian follicles, lung, saliva, oral mucosa, and conjunctiva, but the most abundant group resides in the gastrointestinal tract. This complex community has been referred to as the forgotten organ because of its important relationship with different parts of the organism and its impact on health and disease.
Some microbiota that colonize humans are commensal (non-harmful coexistence), other are mutualistic (mutual benefit) and some are or can be harmful via pathogenic mechanisms or via the metabolites that they produce. For most, the role is not well understood but there is growing evidence of important relationships.
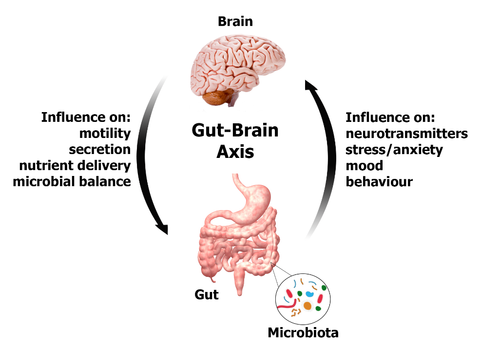
There are also more and more data suggesting a relationship between the microbiota and the central nervous system through different interactions including the release of bioactive compounds. Our intestinal flora can release molecules that effectively and directly influence the brain’s chemistry. There are indications that they can even regulate the production of neurotransmitters, thereby influencing not only our overall health but also our mood and behavior. Thus, the gut microbiome has been shown to regulate the development and functions of the enteric and central nervous systems. Study of the systemic effects of the microbiome has shown profound effects on host peripheral and central nervous systems. These interactions have together been loosely named as the «microbiome–gut–brain axis». One example is the production by Clostridium sporogenes in the gut of 3-indolepropionic acid, a highly potent neuroprotective antioxidant that scavenges hydroxyl radicals. This molecule is absorbed from the intestine and transported to the brain where it confers neuroprotection against cerebral ischemia and Alzheimer’s disease.
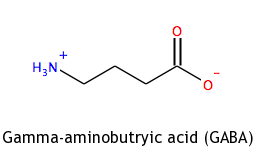
GABA or gamma-aminobutyric acid is the chief inhibitory neurotransmitter in the mammalian central nervous system. It plays the principal role in reducing neuronal excitability throughout the nervous system. This can be the reason why the gut microbiome seems to affect mood. Low levels of GABA are linked to depression and mood disorders. It was demonstrated that administration for 4 weeks of Lactobacillus rhamnosus (JB-1) to healthy male BALB/c mice, promotes consistent changes in GABA-A and -B receptor subtypes in specific brain regions, accompanied by reductions in anxiety and depression-related behaviors. 1 In this study, the researchers found that this effect vanished when they surgically removed the vagus nerve – which links the gut to the brain – suggesting the afferent vagal pathway to the brain may be a significant route whereby beneficial bacteria in the gut might be influencing behavior, although other studies do not find changes after vagus transection.
Recently, the same group examined a standard group of 12 neurometabolites detectable by magnetic resonance spectroscopy (alanine, aspartate, glycine, glutathione, myo/scyllo-inositol, lactate, taurine, GABA, total creatine, total choline, glutamate + glutamine, and total N-acetyl aspartate + N-acetyl aspartyl glutamic acid). 2 This was an attempt to track the neurochemical changes associated with the perturbation of the microbiome–gut–brain axis.
Mice received 1 × 109 cfu of JB-1 per day for 4 weeks and were subjected to MRS weekly and again 4 weeks after cessation of treatment to ascertain temporal changes in these neurometabolites. Delayed increases were seen for glutamate (~10%) and N-acetyl aspartate (~37%) at 2 weeks which persisted only to the end of treatment. However, glutamate was still elevated 4 weeks after treatment had ceased. Significantly elevated GABA (~25%) was only seen at 4 weeks. These results suggest specific metabolic pathways in the pursuit of mechanisms of action of psychoactive bacteria. In addition, the fact that no elevations of abundant biomarkers of brain activity and metabolism including glutamate and N-acetyl aspartate occurred before 2 weeks of treatment, was compatible with the known delays in the clinical therapeutic effects of antidepressants. That GABA was only elevated at 4 weeks is also compatible with this suggestion.
One recent discovery is that some bacteria in the gut are probably fed by the nervous system or at least by typical neurotransmitters. Philip Strandwitz and his colleagues at Northeastern University in Boston discovered that they could only grow a species of recently discovered gut bacteria, called KLE1738, if they provide it with GABA molecules. «Nothing made it grow, except GABA». 3
This group of researchers is nowadays studying other populations of gastrointestinal bacteria that consume or even produce GABA. Then, they will study the effects of these changes in GABA concentration on the neural circuitries and behavior of animals. Associations are beginning to be reported between changes in microbiome profiles in clinical mood disorders, chronic fatigue and neurodegenerative conditions such as Parkinson’s and Alzheimer’s diseases. Hope is that this research on the brain-gut axis may lead to new possibilities for the treatment for these diseases and disorders.
References
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG., …Cryan, J.F (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci 108 (38): 16050–16055. ↩
- Janik R, Thomason LA, Stanisz AM, Forsythe P, Bienenstock J, Stanisz GJ (2016) Magnetic resonance spectroscopy reveals oral Lactobacillus promotion of increases in brain GABA, N-acetyl aspartate, and glutamate. Neuroimage 125: 988-995. doi: 10.1016/j.neuroimage.2015.11.018 ↩
- Coghlan, A (2016) Gut bacteria spotted eating brain chemicals for the first time. New Scientist July 1. ↩
1 comment
They recently tried to recreate these rat/mice experiments in humans and they did not have the same reslults as the mice studies.