Bioorthogonal catalytic activation of anticancer metal complexes
Metal complexes are typically regarded as catalysts that convert organic substrates into more valuable compounds; however, to date, catalytic transformations of metal complexes are practically unknown and represent a complete new way of thinking in catalysis. Their development can expand the scope of bioorthogonal chemical reactions to inorganic substances and metal-based prodrugs, fostering the creation of new inorganic chemistry toolkits for biology and medicine.
Bioorthogonality, a term coined by Carolyn R. Bertozzi in 2003, refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. Hence, catalytic turnover can boost the efficiency of bioorthogonal chemical reactions, unveiling new strategies for prodrug activation and uncaging of molecular probes.
Last year, a team of researchers reported a new type of light-driven reaction in which the exogenous biological molecule riboflavin (Rf) functions as a bioorthogonal photocatalyst and a metal complex acts as an unconventional substrate. This unusual catalyst/substrate pair relies on the photoredox properties of Rf to enable the selective activation of a PtIV prodrug of cisplatin with exceptionally low doses of blue light and induce apoptotic death in PC-3 human prostate cancer cells. Unlike in classic organometallic catalysis, where metals act as catalysts, in this reaction, the metal complex is an unconventional substrate, and the biocompatible riboflavin acts as catalyst.
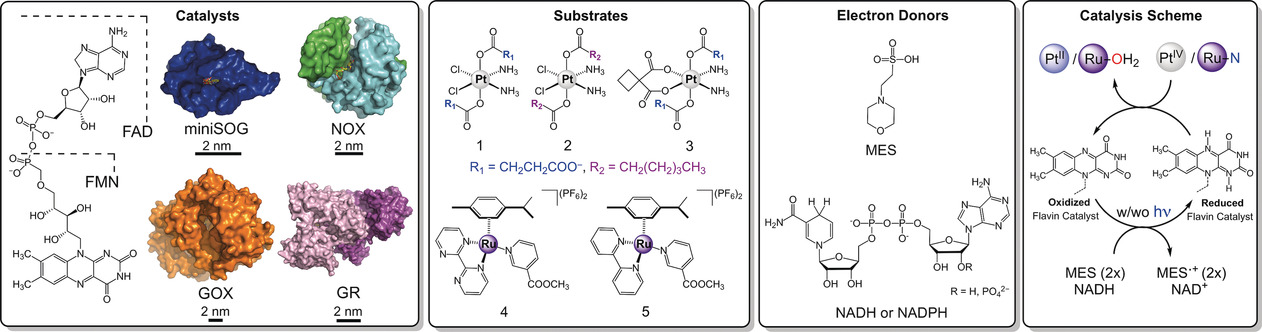
Now, researchers from CIC biomaGUNE and DIPC (some of them participated in last year’s discovery) report 1fundamental discoveries in this new area of bioorthogonal chemistry. To this end they have investigated the catalytic behavior of various flavin catalysts, including four flavoproteins with diverse biological functions and flavin binding pockets. Thay have also increased the pool of inorganic reactions to different PtIV and RuII prodrug complexes, and evaluated the efficiency of different (bio)organic electron donors (Figure 1). Importantly, the results show for the first time that certain flavoproteins may be directly implicated in the activation of metallodrugs under biologically relevant conditions in the absence of light.
In cells, flavins are bound to proteins through covalent and non‐covalent interactions, which control their (photo)redox properties. The team selected four flavoproteins for their diverse flavin‐binding pockets and explored their capacity to catalyze the photoreduction of compounds 1 and 2 (Figure 1). Different chemical environments surround the flavin‐binding pockets in these four flavoproteins, controlling solvent and substrate accessibility to the active site.
The researchers find that, in the presence of electron donors and low doses of visible light, the flavoproteins mini singlet oxygen generator (miniSOG) and NADH oxidase (NOX) catalytically activate PtIV prodrugs with bioorthogonal selectivity. In the presence of NADH, NOX catalyzes PtIV activation in the dark as well.
The discovery of NOX catalytic activity in the dark has broad relevance for understanding the mechanism of action of PtIV anticancer agents, suggesting that flavoproteins can provide alternative and highly efficient activation pathways for metallodrugs. The researchers do not provide a molecular description of the catalytic mechanism through which free flavins and flavoproteins activate PtIV and RuII prodrug complexes, though. In any case, it is clear from the results of this study that protein scaffolds play a crucial role in governing the metal substrate access to the flavin catalytic site.
These findings open new opportunities for the design of chemically and light-activated metal-based chemotherapy drugs, whose biological effects could be triggered endogenously by bioorthogonal flavoprotein catalysts.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Silvia Alonso-de Castro, Aitziber L. Cortajarena, Fernando López-Gallego, Luca Salassa (2018) Bioorthogonal Catalytic Activation of Platinum and Ruthenium Anticancer Complexes by FAD and Flavoproteins Angewandte Chemie international Edition doi: 10.1002/anie.201800288 ↩