Labeling anticancer gold complexes to study their organ accumulation in vivo
Auranofin is a gold (Au) salt classified by the World Health Organization as an antirheumatic agent. It was approved for the treatment of rheumatoid arthritis in 1985, but is no longer a first-line treatment due to its adverse effects on a long-term basis. But the drug is important in another way. The discovery that auranofin has also potent anticancer effects, together with the unceasing quest for metal-based drugs alternatives to cisplatin and its analogues, has stimulated the development of Au complexes as novel chemotherapeutics against cancer. We know now that Au(I) and Au(III) complexes bearing N-heterocyclic carbene ligands (NHCs) display unique anticancer activity profiles in vitro and reduce tumor size in treated mice with transplanted tumoral cells (xenografts).
For Au(III) complexes, reduction to Au(0/I) under physiological conditions has to be considered. Surprisingly, this fundamental aspect has been overlooked in a significant portion of the studies reported on Au(III)-NHCs and their mode of action, generating some confusion on whether Au(III)-NHCs should solely be considered, and hence designed, as prodrugs of active Au(I) complexes. Consequently, our knowledge concerning the fate of Au(III)-NHCs after their administration in vivo is still largely insufficient.
The study of the biodistribution of Au(III) compounds in vivo and the evaluation of their metabolization to Au(I) species is a very challenging task that has not been thoroughly addressed so far, albeit this is a key step in the development of new Au drugs. In this context, a new strategy has been described 1 that exploits Au coordination chemistry for labeling Au(III)-NHCs complexes with radioactive ligands and studying their organ accumulation in vivo.
Au(III) compounds are labelled with iodine-124 so that they can be unequivocally discerned from their Au(I) counterparts and tracked by positron emission tomography. A plus of this new approach is that coordination of iodide has been shown to enhance the anticancer potency of Au derivatives. By combining imaging studies with ex vivo analysis, direct evidence of the metabolization and biodistribution of Au(III)-NHCs overtime can be obtained.
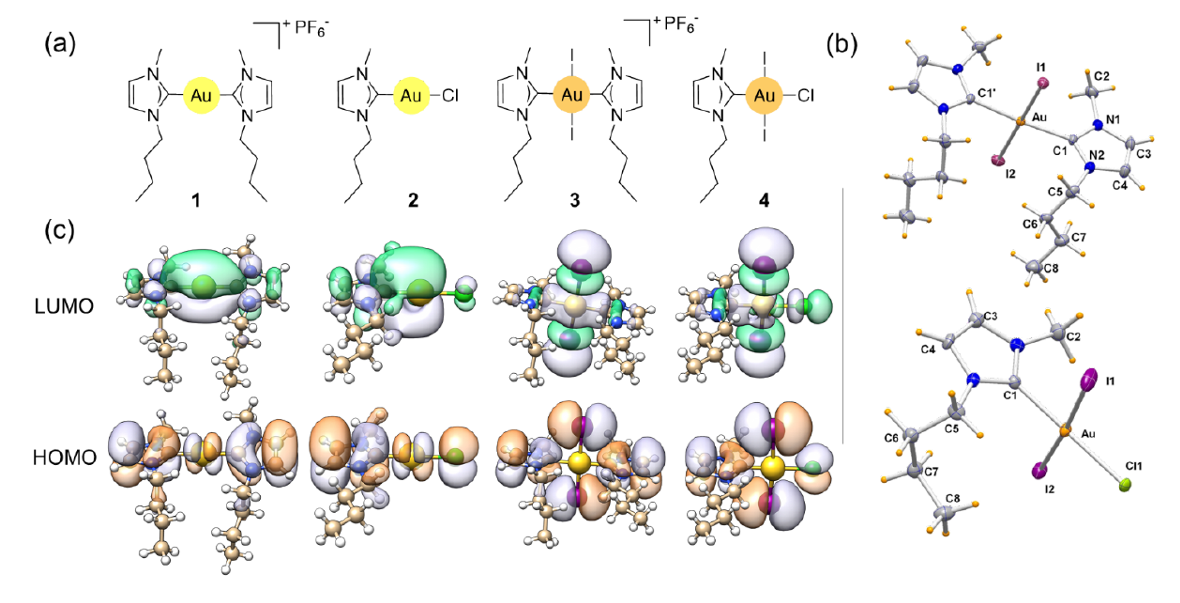
The researchers selected and prepared four Au(I/III) mono and biscarbenic complexes of the 1-butyl-3-methyl-imidazol-2-ylidene NHC ligand (see figure). The Au(I) complexes 1 and 2, have potent in vitro antiproliferative activity against cisplatin-resistant cancer cell lines. The novel Au(III) complexes 3 and 4 were obtained by direct oxidation of 1 and 2, respectively, with elemental iodine.
The team found in vitro a fast Au(III)-to-Au(I) reduction mechanism. Results suggested that fast reduction of 3 to 1 was needed for triggering of the full anticancer activity of the Au(III)-NHC derivative indicating that 3 acts as a prodrug of 1. Additionally, contrary to what reported in the literature for 1 and other similar derivatives, the mode-of-action profile of 3 and 1 strikingly differs from that of auranofin, the reference Au compound to date.
Importantly, while in vitro analyses provided direct evidence for the importance of Au(III)-to-Au(I) reduction to achieve full anticancer activity, in vivo studies revealed that a fraction of the Au(III)-NHC prodrug is not immediatly reduced after administration but able to reach the major organs before metabolic activation. This result urges the medicinal inorganic chemistry community to examine in detail the metabolization of Au(III) compounds.
The new radiolabeling strategy with iodine-124 shows great potentital to unravel the fate of Au(III)-NHC drug candidates in vivo. The same strategy could be applied to study the in vivo biodistribution of other metal-based drugs, including Pt(IV) prodrugs which are currently in different clinical trials.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Federica Guarra, Alessio Terenzi, Christine Pirker, Rossana Passannante, Dina Baier, Ennio Zangrando, Vanessa Gómez, Tarita Biver, Chiara Gabbiani, Walter Berger, Jordi Llop, and Luca Salassa (2020) 124I-radiolabeling of a Au(III)-NHC complex for in vivo biodistribution studies Angewandte Chemie International Edition doi: 10.1002/anie.202008046 ↩