A hydrogel matrix as viable solution for the efficient catalytic activation and delivery of cisplatin
Catalysis-based approaches for the activation of anticancer rely on the use of metal catalysts capable of deprotecting inactive precursors of organic drugs or transforming key biomolecules available in the cellular environment. Nevertheless, the efficiency of most of the schemes described so far is rather low, limiting the benefits of catalytic amplification as a strategy for controlling the therapeutic effects of anticancer compounds. Now, a team of researchers shows 1 that flavin reactivity within a hydrogel matrix provides a viable solution for the efficient catalytic activation and delivery of cisplatin, a worldwide clinically-approved inorganic chemotherapy agent.
Metal complexes, artificial metalloenzymes and nanomaterials can catalyse abiotic reactions in biological environments, both in vitro and in vivo. In the context of chemotherapy, metal complexes have been designed to function as catalysts for the deprotection of organic anticancer agents and for the oxidation/reduction of biomolecules that are key for the cell homeostasis. Something similar happens with metal-loaded catalytic nanocarriers, that have been engineered to be delivered to tumours, or to be placed in their proximity during a surgical procedure, where they can trigger the conversion of prodrugs into their biologically active counterparts.
The great majority of the schemes proposed so far display rather modest catalytic efficiencies, even in buffer solutions, as evidenced by low turnover numbers and slow reaction kinetics. The kinetic aspect is especially overlooked, in spite of its key importance in the development of drug activation strategies. With few exceptions, catalysts currently known typically achieve substrate conversion rates in solution that are in the order of 10-3 to 10-1 min-1. This means that almost any benefit is lost, as this low turnover frequency implies long exposure periods or high loads in order to transform enough prodrug into active drug to induce the desired therapeutic effects. If we now consider the catalyst intrinsic toxicity and that the choice of drugs with high-potency often becomes mandatory, the net result is a limitation in the therapeutic use of catalysis-based strategies.
Flavins and selected flavoproteins photocatalytically convert Pt(IV) prodrug precursors into clinically-approved cisplatin and carboplatin with high efficiency, showing a turnover frequency 25 min-1. Given the exceptional anticancer activity of Pt chemotherapeutics the team speculated that the catalytic efficiency of these flavin-mediated reactions could be the base for a convenient solution for the administration of cisplatin and its derivatives.
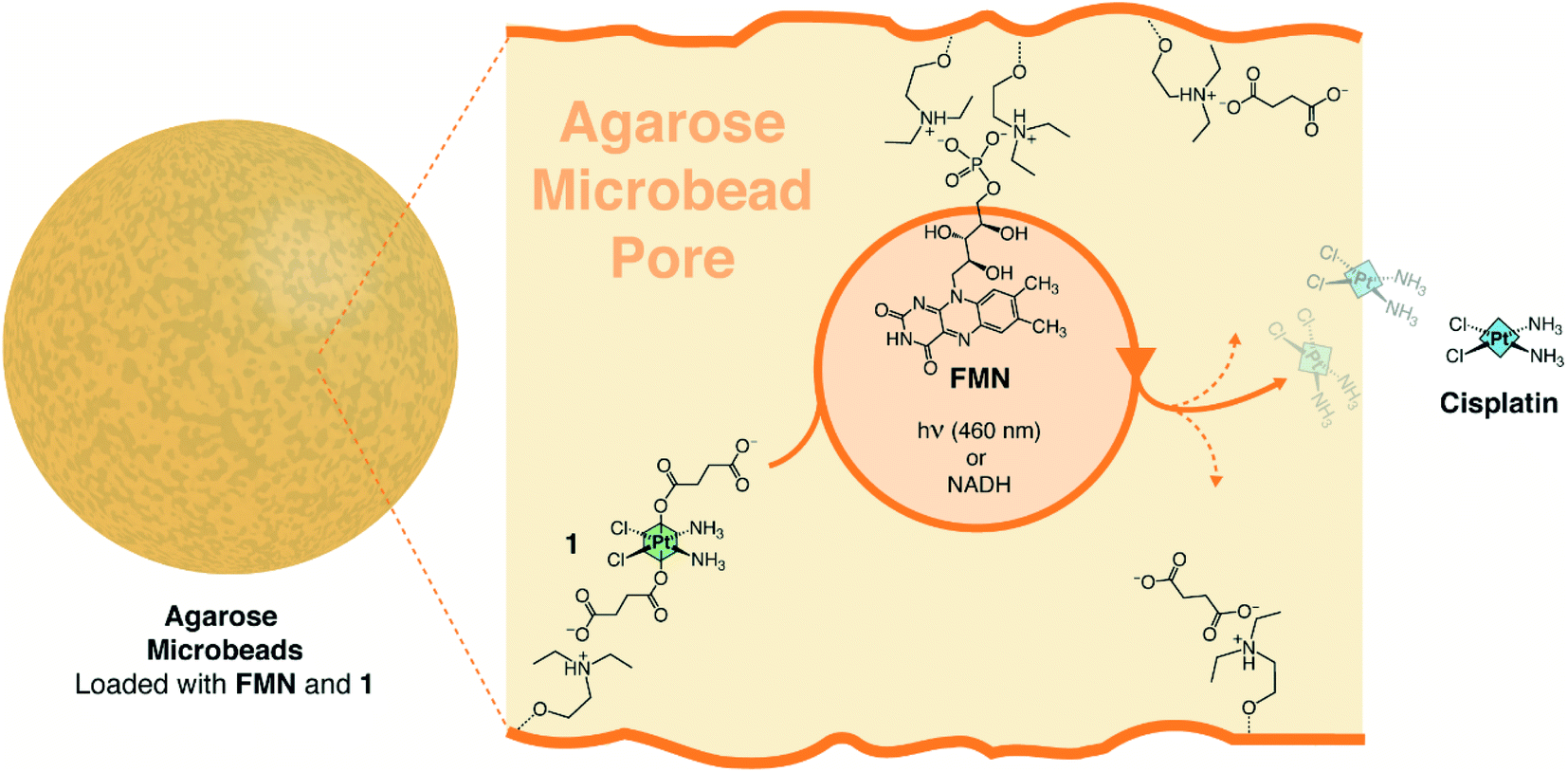
The researchers sought inspiration in nature, which confines biocatalysts (enzymes) and substrates (metabolites) inside organelles to control biosynthetic pathways and boost catalytic performance. This is not new, though, after all, catalysts confined into supramolecular structures have proved very effective in favouring chemical reactions for different technological purposes. However, none of the state-of-the-art systems involve confinement of the catalyst and its corresponding prodrug substrate into an artificial chassis that can operate as a drug depot.
Hydrogels have been widely used as solid supports to immobilize different types of catalysts. These biocompatible soft materials can be applied in surgical implants, locally injected or administered systematically via intravenous infusion, or used to fabricate therapeutic patches for intra- and transdermal release of pharmaceuticals. Stimuli responsive hydrogels introduce an additional level of control on the drug action.
The researchers show how loading of a Pt(IV) prodrug complex and a flavin catalyst onto a hydrogel (agarose porous microbeads) enables performing in situ the confined catalytically-driven generation and subsequent release of cisplatin in response to stimuli, both light and chemical
activation. This is a different approach from the usual, where catalysis has so far been employed to accelerate the degradation of hydrogels and thus control the delivery of the active agents, rather than activating a confined prodrug.
These results may provide a general method to produce remote-controlled high local concentrations of Pt drugs in short bursts. In the long run, this could represent a viable strategy to develop topical medicaments and implantable devices that may overcome some issues associated with the systemic administration of Pt chemotherapeutic agents.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research paper/s.
References
- Susana Velasco-Lozano, Silvia Alonso-de Castro, Carlos Sanchez-Cano, Ana I. Benítez-Mateos, Fernando López-Gallego and Luca Salassa (2021) Metal substrate catalysis in the confined space for platinum drug delivery Chemical Science doi: 10.1039/D1SC05151B ↩