Geometric control of noble-metal nanoparticles
Author: Roberto D’Agosta is an Ikerbasque Research Professor at ETSF and the Department of Materials Physics in the Faculty of Chemistry at the UPV/EHU in San Sebastian. Francesca Baletto, senior lecturer at King’s College London, read and commented the manuscript.

Every single moment, nature exploits catalysis to make or improve specific chemical reactions. Catalysts facilitate specific chemical reactions that would normally not take place without them. A suitable catalyst has many essential features; namely, it should be selective (working for only a class of chemical reactions), resilient to degradation in order to be used continuously, and possibly cheap or readily available.
Unfortunately, for human technological applications the best catalysts are usually developed from either platinum or some noble metal, particularly because of their low level of degradation. To maintain the cost of catalysts reasonable, it is paramount to find a way to improve the efficiency of these catalysts keeping small the quantity used. A possible way to achieve this goal is to design noble metal nanoparticles with certain given conformational properties.
A nanoparticle or cluster is an aggregate of atoms that shows particular properties that are somewhat reminiscent of its components, and yet exceed those of the individual atoms. It has been shown that the geometrical shape of a nanoparticle, i.e. the number of atoms and their actual positions, allows specific chemical reactions. A nanoparticle can indeed offer a multiplicity of facets and local atomic orientation that determine the anchor point of the reactants. Geometry, therefore, plays a decisive role in defining the physical and chemical properties of a nanoparticle.
Building a map between geometrical and physico-chemical factors based on a deep understanding of their relation is paramount for many technological applications, including magnetic recording, chemical reactions, optics, and plasmonics. Moreover, the potential impact of nanoparticle science transcends the boundary of physics and chemistry: a large number of studies are focusing on nanoparticles for drug deliveries in medicine and biology, oil extraction and refinement, weather resistant paints, and since the Middle Ages, the beautiful glasses adorning European cathedrals.
A breakthrough in all these fields consists in providing a geometrical interpretation of the appearance and enhancement of a certain properties in nanoparticles. Indeed, for example, one of the technical challenges lies in designing and assembling metallic nanostructures with controlled geometry increasing the packing density and manipulating their mutual magnetic interaction, e.g., through doping with natural magnets as Fe and Co. Another is studying how the optical spectrum of the nano-particle is affected by the atomic arrangement.
Recent progress has been made in this direction by a group of researchers lead by Dr Francesca Baletto at King’s College London 1 2. These works attempt to characterise, via ab-initio methods, the relation between shape and magnetism. Moreover, the presence of an external environment, for example, a zeolite framework or hydrogen on the surface of the nano-particle, plays a decisive role in further tailoring the desired properties. In particular, it has recently been shown that the geometrical shape of a platinum nanoparticle can determine its magnetic response [1]. Platinum in bulk is usually a non-magnetic material, but at the nanoscale the absence of any translational invariance changes completely the picture and almost all these noble metal nanoparticles present a ferromagnetic behaviour, see Figure 1.
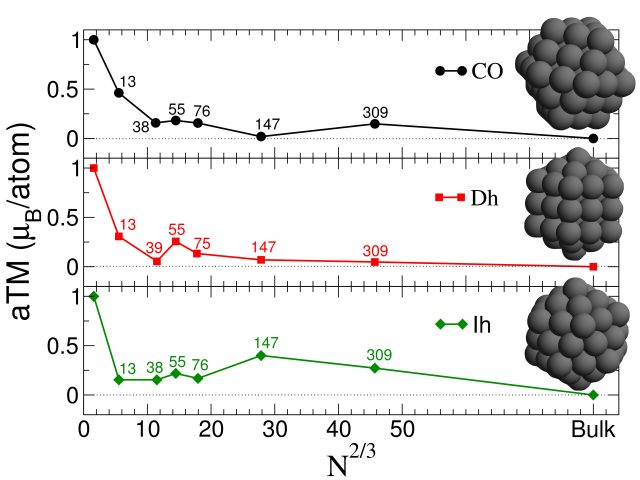
Indeed, the presence of local strain between the external atomic shell and the first atomic sub-shell of the clusters induces a charge transfer from the latter to the former. The presence of isolated vertexes, where the charge and spin accumulate, further increases the magnetic response of the cluster. Therefore the presence of strong ferromagnetic response has been correlated with the presence of elongated and contracted distances, beyond the typical argument based on the presence of certain high symmetry axes.
A fundamental question on nanoparticles used as catalyst is the investigation on how the presence of the external environment influences the response of the cluster. In particular, zeolites – generally a silica framework regularly in use in many chemical industries for various technological applications – constitute a useful playground to understand how the cluster binds to the framework. For example, before some work on the topic [2], it was not clear to which atomic configuration the platinum clusters do bind. Also, it was shown how a large family of clusters can fit into the geometrical restrictions imposed by the shape of the zeolite: previous work considered only highly symmetric shapes which however do not fit into the main pore of the zeolite and are unstable in that configuration.
This study entailed a diversity of approaches: on the one hand, starting with a given number of atoms in the cluster, which was fixed to 13 by recent experiments, a search of all the optimal classical geometries was performed via a meta-dynamics formalism. This method allows for a fast sampling of the free energy surface with the identification of the stable and metastable configuration. On the other hand, ab-initio methods have been used to verify the stability of the local minima found with meta-dynamics and to characterise their magnetic response. Only those clusters that fitted in the geometrical constraints of the zeolite cage have been further investigated. It was found that the clusters do preferentially bind to a site of the zeolite and subsequently they undergo a structural reconstruction to minimise the total energy. Only then the magnetic response was considered. Finally, it was investigated how the presence of hydrogen adsorbed on the cluster modifies both the anchor point, the cluster orientation, and the magnetic response providing a useful tool to identify the hydrogenation state of a cluster in the zeolite.
Numerical tools offer an unprecedented way to understand nanoscale systems to explain and design nanostructures with tailored physical and chemical properties. Understanding how geometrical shapes control these properties is paramount to our technology since it reduces the amount of wasted material – this is particularly important when working with noble metals since they are increasingly scarce on our planet. Platinum in this respect shows outstanding catalytic and magnetic properties whose control and tailoring offer secure advances for many technological applications including oxygen reduction in hydrogen batteries and magnetic recording, to name just two.
References
- Geometrical effects on the magnetic properties of nanoparticles, by Cono Di Paola, Roberto D’Agosta, and Francesca Baletto, Nano Letters 16, 2885 (2016). doi: 10.1021/acs.nanolett.6b00916 ↩
- Structural stability and uniformity of magnetic Pt13 nanoparticles in NaY zeolite, by Cono Di Paola, Luca Pavan, Roberto D’Agosta, and Francesca Baletto, Nanoscale 9, 15658 (2017). doi: 10.1039/C7NR03533K ↩