Single-chain nanoparticles, a world of opportunities
One of the unique features of the chemistry of carbon (and, to some extent, silicon) is its ability to form long chains of atoms. Polymers are substances that have macromolecules composed of many repeating units (known as ‘mers’). Many naturally occurring substances are polymers, including rubber and many substances based on glucose, such as the polysaccharides cellulose and starch (in plants) and glycogen (in animals). Proteins, nucleic acids, and inorganic macromolecular substances (like polysiloxanes, commonly called silicones, or silicates) are other examples.
One of the features of some of these natural polymers is that their overall three-dimensional structure may vary. Take the case of proteins. Proteins comprise carbon, hydrogen, oxygen, and nitrogen and most also contain sulphur. Protein molecules consist of one or several long chains (polypeptides) of amino acids linked in a characteristic sequence. This sequence is called the primary structure of the protein. These polypeptides may undergo coiling or pleating that results in the secondary structure. The three-dimensional shape of the coled or pleated polypeptides is called tertiary structure. There is still a quaternary structure, which is the structural relationship of the component polypeptides. These two last structures are key to the functionality of the protein.
There is a rough analogy between the process of single-chain nanoparticles formation and the precise, specific folding of a polypeptide chain to its native, functional state. Single-chain nanoparticles are the result of folding individual synthetic polymer chains at high dilution by means of multiple intra-chain (reversible or irreversible) bonding interactions. Most single-chain nanoparticles result in a typical morphology in solution more akin to those displayed by intrinsically disordered proteins than those found in high-functional proteins like compact, globular enzymes.
Folding a synthetic polymer chain restricts significantly its conformational degrees of freedom as exemplified by cyclic polymers. Upon ring closure through bonding of chain-ends in a linear chain, a great number of important properties drastically alter when compared to those displayed by the analogous uncycled macromolecule, such as density, refractive index, glass transition temperature, crystallization behaviour, hydrodynamic size and viscosity, as well as surface energy.
Cyclotides are a natural case on point. They consist on cyclic polypeptides stabilized by intra-ring disulphide bonds, as naturally occurring in a variety of bacteria, fungi, and plants as well as animals. Because of their folded structure, cyclotides show remarkable properties in comparison to equivalent non-cyclic polypeptides such as improved enzymatic stability against proteases or increased bacterial cell membrane disruption capability.
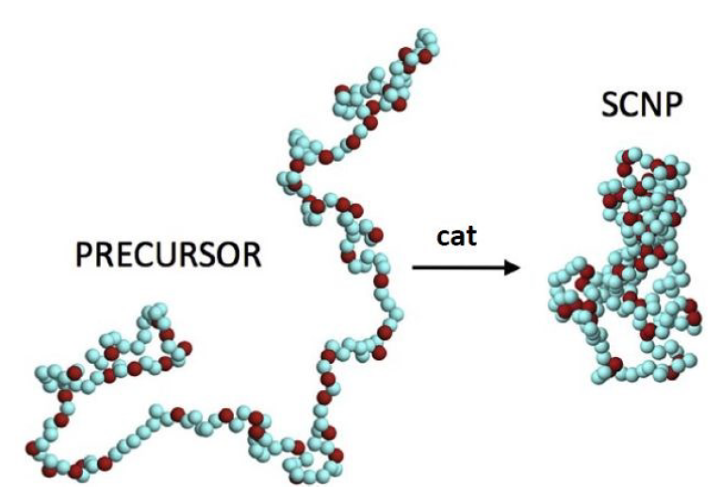
Likewise, the conformational degrees of freedom in single-chain nanoparticles are severely restricted giving rise to notorious local domain formation and, hence, to topological self-confinement effects –a class of topological effect resulting from the folding of a linear synthetic polymer chain to a single-chain nanoparticle. Interestingly, these effects are beneficial for the construction of efficient enzyme-mimetic catalysts, improved drug delivery nanosystems and innovative sensing nanomaterials, as a recent review article 1 shows.
In this article, after considering the opportunities that internal confinement in single-chain nanoparticles offers for catalysis; encapsulation, transport and delivery of therapeutic cargos; sensing, targeting and bioimaging applications as well as other diverse advanced applications, the authors try to provide a unifying vision of what is the expected effect of external confinement – as exerted over single-chain nanoparticles by different geometrical constraints as well as in solution, in melt state, on surfaces and in membranes- on the size, shape and, hence, potential functionality / foreseen applications of single-chain nanoparticles.
Some of these potential uses, especially those related to self-confinement, have already been established. Importantly, the authors anticipate a plethora of opportunities that open up arising from external confinement of single-chain nanoparticles: from innovative purification techniques and improved all-polymer nanocomposites, to smart responsive surfaces and new topological nanostructures.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
Disclaimer: Parts of this article may be copied verbatim or almost verbatim from the referenced research paper.
References
- Ester Verde-Sesto, Arantxa Arbe, Angel J. Moreno, Daniele Cangialosi, Angel Alegría, Juan Colmeneroabc and José A. Pomposo (2020) Single-chain nanoparticles: opportunities provided by internal and external confinement Materials Horizons doi: 10.1039/D0MH00846J ↩