Origin of the mysterious blue fluorescence of polymer carbon dots
Origin of the mysterious blue fluorescence of polymer carbon dots
A quantum dot is a nanometric crystalline structure of semiconductor materials. In a quatum dot electrons are confined in a region of space, thus creating a well defined structure of energy levels that depends very much on the size and shape of the quantum dot. This structure resembles that of atoms, that is why sometimes quantum dots are also called artificial atoms. One variety is that of carbon spherical nanoparticles (less than 10 nm in size), the so-called carbon dots. A unique feature common to the vast variety of polymer carbon dots prepared from carboxylic acid and amine precursors is a mysterious emission of a bright blue fluorescence.
Fluorescent carbon dots are present in the most recent advances in the fields of sensors, biosensors, bioimaging, drug delivery, light emitting devices, and photocatalysis. This widespread use of carbon dots is due to their straightforward fabrication from a broad set of cheap precursor materials, on top of their water solubility, low toxicity, and good biocompatibility. Somehow paradoxically, the difficulty to ascertain a precise chemical structure means that our understanding of the general fluorescence phenomena is lacking and hinders the design of advanced carbon dots with tunable optical properties.
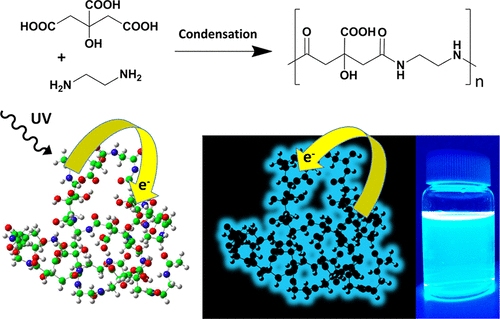
A team of researchers, including Jon Matxain (UPV/EHU & DIPC), has built 1 highly blue fluorescent model types of carbon dots from citric acid and ethylenediamine through low temperature synthesis routes. Density functional theory calculations of these model carbon dots uncover the origin of the strong blue fluorescence emission.
The synthesis of fluorescent carbon dots by common bottom-up approaches involves the polymerization of organic molecules or the cross-linking of polymers at high temperature in hydrothermal or microwave-assisted reactions. The formation process resembles a dehydration and condensation process among the abundant functional groups of the precursor materials. As a result the polymer carbon dots are composed of mixtures of molecules, oligomers, polymer chains and polymer clusters. In other words, random reaction processes lead to a vast number of products with a unique blue fluorescence emission as the only common unifying feature.
In order to explain this flourescence some have said that it is related to conjugated fluorophores, while others have pointed out that also non-conjugated polymers cause a bright photoluminescence, regardless of the presence of other emissive sources such as molecular fluorophores or a carbon core, ascribing the fluorescence to the so-called cross-link enhanced emission effect. The point is that it is difficult to give a convincing explanation to a phenomenon when a precise chemical structure can not be assigned.
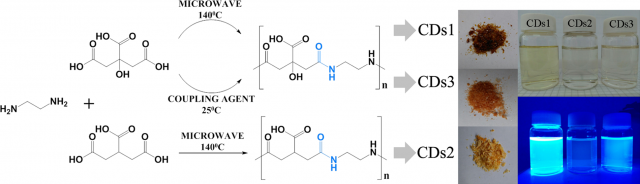
So, the researchers set out to unambiguously identify the origin of the blue fluorescence, commonly observed in non-conjugated polymer carbon dots. To this end, model types of blue fluorescent carbon dots with a well-defined structure, consisting of commonly employed carboxylic acid and amine functional groups, were synthesized. Citric acid and ethylenediamine were chosen as they are rather simple precursor molecules. Three different synthesis routes were designed to establish a polycondensation process resulting in amide bond formation while avoiding undesired side-reactions. This includes low temperature microwave assisted reactions controlling reaction time and reactive groups, as well as a novel yet unexplored room-temperature synthesis route, exploiting reactions based on carbodiimide chemistry. All three routes produce highly blue emitting fluorescent polymer carbon dots with diameters in the 1−1.5 nm range.
The density functional theory (DFT) analysis of the ground and excited states of these model polymer carbon dots discloses the existence of spatially separated highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbitals located at the amide and carboxylic moieties, respectively. The photoinduced charge transfer between these groups would be then the origin of the strong luminescence behaviour.
These photoinduced charge transfer processes in the polyamide network structure easily explain the performance of carbon dots in technological applications as revealed in studies on metal ion sensing. More importantly, this result paves the way to exploit novel synthetic bottom-up routes to obtain polymer carbon dots with tailored properties, likely uncovering new opportunities.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Lorenzo Vallan, Esteban P. Urriolabeitia, Fernando Ruipérez, Jon M. Matxain, Ruben Canton-Vitoria, Nikos Tagmatarchis, Ana M. Benito, and Wolfgang K. Maser (2018) Supramolecular-Enhanced Charge Transfer within Entangled Polyamide Chains as the Origin of the Universal Blue Fluorescence of Polymer Carbon Dots J.A.C.S. doi: 10.1021/jacs.8b06051 ↩
1 comment
[…] Read the full story here! At “MappingIgnorance.org”, written by César Tomé López, science writer and the editor of Mapping Ignorance. […]