Spin in a closed-shell organic molecule stabilized on a metallic surface
Every year the amount of data produced is of the order of magnitude of the Avogadro’s constant, thus 6.028×1023. This trend is supposed to increase even more in the next future. This implies that more and more special metals will be needed. The point is: what might happen if the resources used for manufacturing common storage devices are exhausted, their prices sky-rocket or their reserves become controlled by a supervillain? We need an alternative. Enter molecular electronics.
For example, many aromatic compounds can be made into organic semiconductors by doping them with a substance such as iodine, thereby producing mobile carriers of electric charge. This is analogous to the doping of silicon in an ordinary semiconductor. The benefits of using organic compounds are evident, namely, the resources are available everywhere, extremely cheap, and there is a great versatility for synthesizing the different compounds. So much so, that some of these organic compounds present magnetic properties, like the ones present in an iron-based magnet.
In spintronics, new memory and logic devices are being developed based on the use of spins of nuclei, atoms, or molecules, instead of electronic charges. The main advantages are an improved energy efficiency and speed of operation to store and process information. The ability of organic molecules to maintain magnetic multistability in nanoscale junctions will determine their role in downsizing spintronic devices.
Thus, it is desirable to design sustainable pure organic magnets to replace metals. Potential candidates are spin ½ molecular free radicals, i.e., a group of atoms with an unpaired valence electron, what makes them extremely reactive. This reactivity represents a huge problem when we try to manipulate free radicals as any change transfer ruins spin control. Inert metal surfaces, like that of gold, present more chances of hosting organic intact radicals than other substrates, but large amounts of delocalized electronic states favour charge transfer and thus spin quenching. Lowering the molecule–substrate interaction with insulating layers is a usual strategy to stabilize radicals on surfaces.
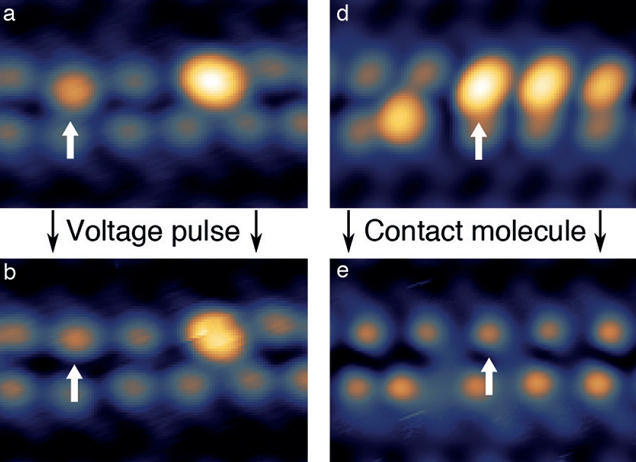
Recently, something unexpected was reported. The closed-shell (not a radical) molecule retinoic acid (ReA,) was deposited onto a crystalline gold(111) surface and then subjected to current pulses with a scanning tunneling microscope (STM) (Figure 1). This procedure reproducibly led to intriguing changes in the molecule. The apparent height of the cyclic end of ReA (the“head”) increased by 140 pm and the head group appeared laterally shifted while the rest of the molecule (the “tail”) remained virtually unaltered. Surprisingly, this modification made the molecule to exhibit a localized spin. As expected for a localized spin, this feature could be tuned by an external magnetic field. These results suggest that it may be possible to bottom-up assemble arrays of switchable spins on surfaces.
But, wait a minute. How on earth does it happen if there is no radical to start with? Because the calculations were unable to find a radical on the surface that could explain the experimental data.
Now, a team of researchers that includes Nicolás Lorente (CFM & DIPC), presents 1 a reaction pathway (not reported on surface chemistry) that leads to spin localization in ReA on a metal substrate as observed in the STM experiments. The final reaction product is a neutral radical.
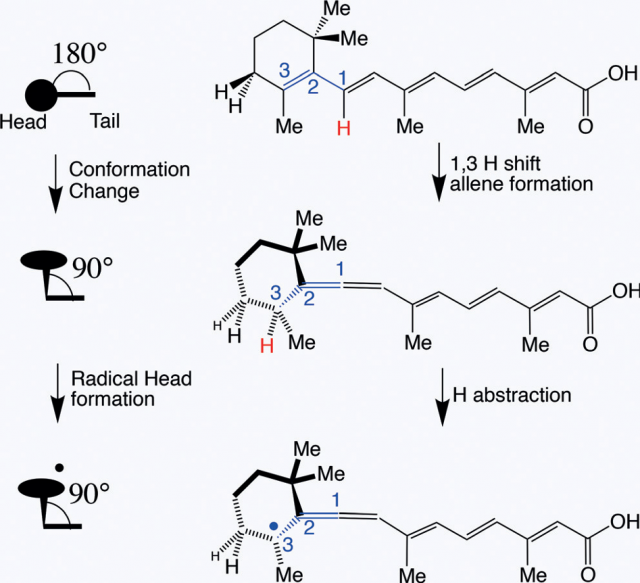
The radical is formed (Figure 2) by loss of a hydrogen atom from an allene intermediate that is produced by a concerted hydrogen sigmatropic rearrangement of the ReA molecule, one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular process. The proposed mechanism is corroborated by density functional theory calculations and STM contact measurements allowing reversible switching and it is consistent with all experimental observations.
The use of local probes to induce reactions on organic layers allows an enhanced control of charges and spins at the interface between a molecular layer and a solid substrate. This means that organic radicals may be stabilized on metallic surfaces without resorting to insulating layers. A search for molecules that may be switched on surfaces to a state with a localized spin is in progress.
Author: César Tomé López is a science writer and the editor of Mapping Ignorance.
References
- Marie-Laure Bocquet, Nicolas Lorente, Richard Berndt, and Manuel Gruber (2018) Spin in a Closed-Shell Organic Molecule on a Metal Substrate Generated by a Sigmatropic Reaction Angewandte Chemie International Edition doi: 10.1002/anie.201812121 ↩