Mapping PTEN: basic research to assist precision oncology
Author: Rafael Pulido is an Ikerbasque Research Professor at the BioCruces Health Research Institute
In the last two decades, the easiness in the obtaining of genetic information from patient biological samples, together with the advanced knowledge on the physiological consequences of patient genetic alterations, has transformed the daily clinical practice in our hospitals. As a result, the current use of the precision medicine concept, as disseminated today in the specialised and general media, is mainly based on the accessibility to genetic information from the patient.
However, precision medicine should be subjacent to some other clinical considerations in the management of a patient, beyond the molecular status of his/her genes. Among these, a precision diagnostic is essential, which is not necessarily driven by the patient genetic information, and which can rely on the appropriate use of a precision reagent -for instance, a specific antibody-, as an informative clinical tool. A patient diagnosed precisely will be likely treated with a precision drug (if known and available) which will target the specific molecule or biological activity causative of the disease, reverting its pathological effects.
In clinical oncology, historical examples of successful precision medicine exist before the genomic era entered in our advanced societies. A landmark at this respect is the beneficial use of tamoxifen to treat women with estrogen receptor-positive breast cancer, a therapy which was approved about 40 years ago, when the human genome was still a black box under the pursuing of no one. The presence of estrogen receptor on breast cancer cells, as detected by immunohistochemistry using anti-estrogen receptor monoclonal antibodies, determines the treatment of the patients with tamoxifen. Monoclonal antibodies are precision reagents with unique specific recognition towards the proteins they bind to, and they are now routinely used in research and in clinical oncology to detect the presence of specific proteins of clinical interest -biomarkers- in tissues and tumors.
Tumor suppressors are proteins which defective function associates with cancer predisposition and tumor development, the PTEN protein being one of the tumor suppressors more relevant in human cancer. The gene that encodes PTEN is frequently absent or mutated in human tumors, and its inherited alterations are causative of tumor predisposing syndromes. In addition, many non-genetic factors influence the amount and function of PTEN in cells and can also be related with tumor initiation and progression 1. This makes the determination of PTEN protein presence in tumors, or PTEN protein levels in biological samples, potential tests to predict cancer and its evolution in patients, and to decide an appropriate therapy. However, in spite of its importance as an anti-tumor protein, there are not yet officially approved tests to measure PTEN presence or functionality in patient samples, that could help to oncologists in the treatment of cancer. Several anti-PTEN monoclonal antibodies exist that can be used with diagnostic purposes, although most of them have been poorly characterized when considering which part of the protein -which epitope- they bind. To address this issue, we have performed a meticulous work that maps with high precision the short linear epitopes at the end of the PTEN protein that are recognized by anti-PTEN antibodies 2 (Figure 1).

Importantly, our study has unveiled not only the immunodominance of the final region of PTEN to be targeted by the studied antibodies, but also that specific modifications occurring in PTEN protein under physiologic or pathologic circumstances may abrogate PTEN recognition by these specific reagents. Such modifications include amino acid changes in the protein created by PTEN genetic mutations found in tumors and in patients, as well as alternative splicing-derived truncations, phosphorylation, or proteolytic modifications not linked directly to a genetic alteration. Some of these changes have been found not to affect severely the tumor suppressor function of PTEN, at least in experiments performed in the laboratory, but they impede the antibody binding.
These findings serve as the proof-of-concept that there is a necessity to improve our basic knowledge on the precise reactivity of antibodies used in clinical oncology, to help in the obtaining of a precise clinical diagnostic based on such reactivity (Figure 2).
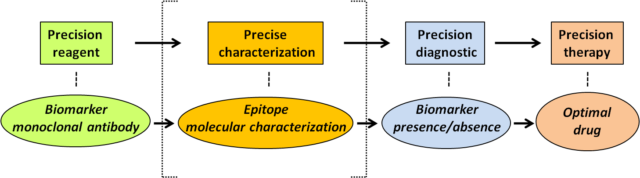
In other words, to provide accurate information on the presence of a protein of interest in clinical oncology, based on antibody recognition, it would be convenient not only that the antibody displays good sensitivity and specificity towards the biomarker of interest, but also to have a precise characterization of the epitope recognized by the antibody on the biomarker protein, and of the physiologic or pathologic conditions in which this epitope could be lost without affecting the presence of the protein.
The precise characterization of the epitopes recognized by antibodies recognizing clinically-relevant proteins is a dedicated and laborious task which, in many cases, is overlooked by researchers. Our work now reported on the precise epitope mapping of the tumor suppressor PTEN should be a notice for researchers and clinicians that there are still basic aspects to investigate when it comes to the use of antibodies as precision reagents that may assist the clinicians in therapeutic precision oncology decisions.
References
- Pulido, R. (2015) PTEN: a yin-yang master regulator protein in health and disease, Methods, 77-78, 3-10. ↩
- Mingo, J., Luna, S., Gaafar, A., Nunes-Xavier, C.E., Torices, L., Mosteiro, L., Rebeca Ruiz, R., Guerra, I., Llarena, R., Angulo, J.C., López, J.I., Pulido, R. (2019) Precise definition of PTEN C-terminal epitopes and its implications in clinical oncology. NPJ. Precis. Oncol. doi: 10.1038/s41698-019-0083-4 ↩
2 comments
Very interesting perspective for all antibody-based diagnostic techniques; among the several flaws of immunoassays and other detection methods developed from them, not very often we think about the molecular details underlying the specificity of antibodies, and it is very interesting to evaluate the usefulness of such methods when reactivity is compromised without a reflection on protein function or structure… a really interesting perspective.
It is curious that even research in PTEN has been directed towards the predominant “precision medicine” tag… nobody escapes.
Great job and great review, boss! ;D
“After getting the sign to enter the hut, the young woman went inside and softly let on the shaman’s arms the fur wrapped sick baby she was carrying. The shaman stood up, laid the small pack on a low table and made a careful visual examination of the fragile body inside the mantle. The baby cried. The shaman stretched the baby legs, pressed its belly, and fingered its neck as in a routine procedure. He asked something to the mother. She answered. He went back with special attention to the baby’s ears. Finally, he rubbed meticulously his hands and put his left palm first on the baby’s forehead, second on its nape. The shaman nodded, wrapped warily the baby and gave it back to the mother. The silence came back to the hut. The shaman put on the fire a small saucepan with water, added a handful of dry willow leaves, and let it to boil. Soon, the mother came out of the hut with the baby, a pumpkin canteen filled with the infusion, and some instructions on how to give the medicine to the little one so the fever will go away” (a brief account of precision medicine in the early days).
Precision medicine always existed: it is just a question of how precise the shaman wants to be, which is usually inversely proportional to his/her ignorance. The less we ignore, the better.
Thanks!