In situ recording of in vivo analyte biodistribution using X-ray fluorescence imaging
There are instances in which you would intentionally deliver nanoparticles to animals (humans included). Contrast agents for medical diagnosis or therapeutic ones for medical treatments are two examples. It is also the case with plants, as some fertilizers take the form of nanoparticles. Unintentional exposure of animals or plants to nanoparticles is also possible: environmental pollution being the most common scenario. The biodistribution of nanoparticles in an exposed organism as it changes with time and how nanoparticles interaction with the organism affects that distribution are important variables to consider to understand risks and possible outcomes of the exposure.
Even though a variety of imaging techniques (fluorescence imaging, magnetic resonance imaging, computed tomography, single photon emission computed tomography, mass spectrometry imaging) can be used to get this level of analysis to some extent for nanoparticles that provide the appropiate contrast, there are clear limitations in terms of suitable nanoparticles, in imaging specimens with macroscopic dimensions, and in the potential damage to or destruction of the sample during measurements. These limitations are apparent in the literature about the subject. Most of the published work, dominated by animal studies, involves sacrificing animals during or after exposure, and thus, the number of animal “samples” that can be analyzed is inherently limited.
However, on the other hand, the same restrictions do not apply for imaging biodistribution in plants. Plants are complex biota but do allow for in situ and in vivo experiments without the restrictions imposed by animal species. Actually, the ability to monitor nanoparticle biodistributions in vivo would significantly advance cutting-edge areas of research such as nanoscale mediated genetic transfection, ecotoxicity induced by nanoparticulate matter or the development of new nanoenabled precision agrochemicals.
The effect of nanoparticles exposure will depend on the biodistribution of the nanoparticles inside the plants. Nanoparticles which remain stuck in the roots for example would have a different effect than nanoparticles which are transported up to the petals. Thus, recording nanoparticle biodistributions in plants is an important topic by itself. Interestingly, in situ techniques developed for the in vivo imaging of nanoparticle biodistribution in plants could later be extended to, and modified for, in vivo imaging of animal species.
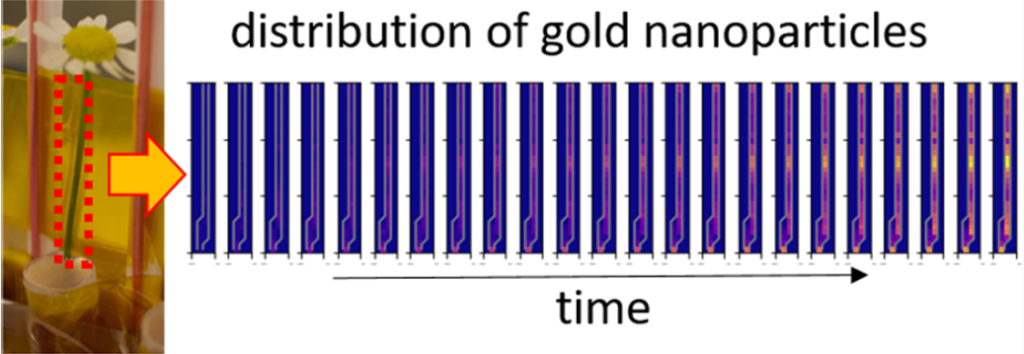
In this line of research, a team of researchers investigated 1 the potential of synchrotron-based X-ray fluorescence imaging (XFI) for mapping the in vivo biodistribution of nanoparticles by real-time tracking in plants to enable a dynamic “pharmacokinetic movie”.
Analogous to fluorophores in the IR/visible/UV, all elements except hydrogen and helium have distinct X-ray fluorescence and thus, can be spectrally distinguished. However, scattering in biological tissue is the primary hurdle for recording optical fluorescence. Given that this scattering is not isotropic, the background can be reduced by application of appropriate algorithms. In this way, XFI can be used to probe nanoparticle fate through deep tissue imaging. While radiation damage must be considered, effective non-destructive imaging is possible, allowing potential real-time in situ/in vivo biodistribution measurements and mapping.
To demonstrate the feasibility of this approach, the researchers developed a model system based on the popular ink flower experiment that involves a daisy flower being placed in a pot of ink, with subsequent visualization of the distribution of the pigment within the petals as a color change. Instead of a daisies (Bellis perennis), the team chose the easily available camomiles (Matricaria chamomilla) as model flowers bacause in the upper part of their stem (the last 4 cm) there are almost no leaves, and stem, petals, and blossom could be easily distinguished as separated compartments. The part of the ink was given to gold nanoparticles, as this material allows for creating defined libraries in which size and surface chemistry may be varied.
Camomile flowers were incubated with gold nanoparticles of different sizes ranging from 1.4 to 94 nm. After different incubation times of 6, 12, 24, and 48 h, the gold distribution in the flowers was destructively measured by inductively coupled plasma mass spectrometry (ICP-MS) and non-destructively measured by X-ray fluorescence imaging (XFI) with high lateral resolution. As a control, the biodistribution of iodine ions or iodine-containing organic molecules (iohexol) was determined, in order to demonstrate the feasibility of mapping the distribution of several elements in parallel.
The results show clear size-dependent transport of the nanoparticles. In addition, the surface chemistry also plays a decisive role in disposition. Only the 1.6 nm nanoparticles coated with acetylcysteine could be efficiently transported through the stem of the flowers into the petals. In this case, almost 80% of the nanoparticles which were found within each flower were located in the petals. These findings validate the potential of XFI for in situ recording of in vivo analyte biodistribution.
More on the subject:
Labeling anticancer gold complexes to study their organ accumulation in vivo
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research papers.
References
- Yang Liu, Christian Körnig, Bing Qi, Oliver Schmutzler, Theresa Staufer, Carlos Sanchez-Cano, Elisabeth Magel, Jason C. White, Neus Feliu, Florian Grüner, and Wolfgang J. Parak (2022) Size- and Ligand-Dependent Transport of Nanoparticles in Matricaria chamomilla as Demonstrated by Mass Spectroscopy and X-ray Fluorescence Imaging ACS Nano doi: 10.1021/acsnano.2c05339 ↩