Producing a large quantity of pure cyclic polymers
Cyclic polymers present a topology that differ significantly from their linear counterparts due to their circular structure and, therefore, the lack of chain ends. These simple characteristics are responsible for important unique properties (e.g. lower intrinsic and melt viscosity, lower hydrodynamic volumes, slower degradation profiles, increased blood circulation times and more selective bioaccumulation) thanks to which the cyclic polymers are today a vanguard in macromolecular chemistry.
However, the preparation of cyclic polymers with high topological purity and in large quantities is challenging, demanding new synthetic methods. What brings us to the Nobel Prize in chemistry 2022, that recognizes the work of Morten Meldal and Barry Sharpless. They, independently of each other, developed an elegant and efficient chemical reaction that is now in widespread use and is utilized in the development of pharmaceuticals, for mapping DNA and creating new materials. This is now a field called “click” chemistry.
In 2001 the concept of click chemistry was introduced by Sharpless et al. In order to be qualified as “click” reaction, the reaction must be modular, very high yield and wide in scope. In the case of the formation of by-products, they must be inoffensive and removable by non-chromatographic methods. Additionally, the reaction conditions must be simple (i.e. readily available reagents, easily removable solvent and purification by non-chromatographic methods). Finally, the product must be stable under physiological conditions. In 2002, Fokin, Sharpless et al. and Meldal et al. reported simultaneously the use of a copper (I) catalyst in the cycloaddition of azides and alkynes, allowing the reaction to proceed at room temperature. Since then, the copper-catalyzed azide alkyne cycloaddition (CuAAC) has been extensively used in polymer chemistry. This use of click chemistry constituted a great breakthrough for the synthesis of cyclic polymers.
As mentioned above, the absence of end groups makes the cyclic polymers unique. And not only that, the absence of end groups makes a cyclic polymer to have lower conformational degree of freedom and more compact coil conformation compared to their linear analogs. They are characterized by a reduced melt viscosity, a reduced entanglement, and lower hydrodynamic volume. These features could be very useful in medicine, nanotechnology and material science.
Now, a team of researchers reviews 1 the most recent advances in the design and synthesis of cyclic polymers by compiling already known concepts of promising strategies and recent synthesis milestones. The strategies considered are ring closure (RC) and ring expansion polymerization (REP) techniques, which are today more attractive than the original ring-chain equilibration for the synthesis of well-defined macrocyclic structures due to their versatility and purity of the obtained products.
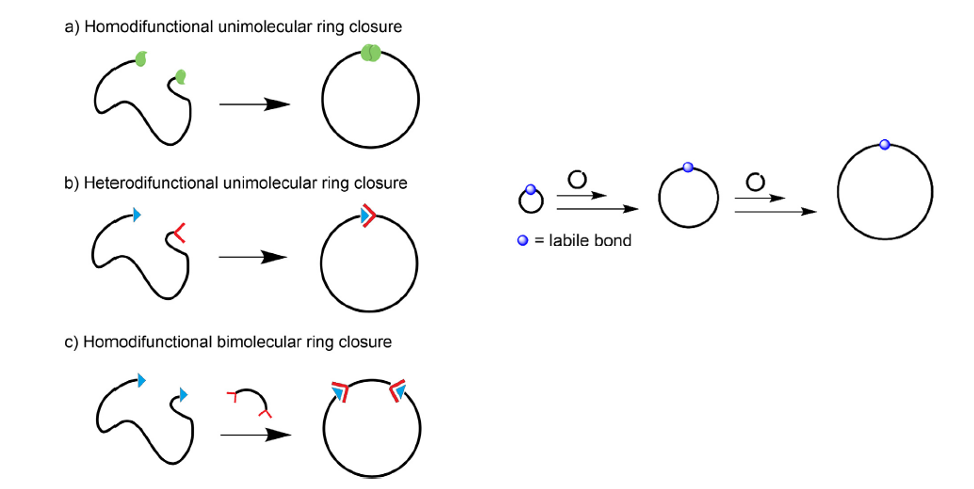
The RC strategies (see Figure 2) rely on intramolecular coupling of the end-groups of a previously synthesized linear precursor. The use of predesigned linear polymers as precursors for the preparation of cyclic polymers allows high control over the molecular characteristics of the obtained rings. REP allows the synthesis of “specific” cyclic polymers via the formation of an initial ring that expands upon the incorporation of monomer units through a weak labile bond (e.g. organometallic or electrostatic). “Specific” meaning that only the right combination of monomer and catalyst would produce the desired cyclic structure. At the end of the reaction, the catalyst is either retained or expelled from the macrocycle.
The team concludes stressing the difficulty in producing a large quantity of pure cyclic polymers with current methods. This is the obstacle behind their little interest to industry, for the time being. New improved synthetic and purification techniques are needed.
More on the subject:
Author: César Tomé López is a science writer and the editor of Mapping Ignorance
Disclaimer: Parts of this article may have been copied verbatim or almost verbatim from the referenced research papers.
References
- Jordan Ochs, Carlo Andrea Pagnacco, Fabienne Barroso-Bujans (2022) Macrocyclic polymers: Synthesis, purification, properties and applications Progress in Polymer Science doi: 10.1016/j.progpolymsci.2022.101606 ↩